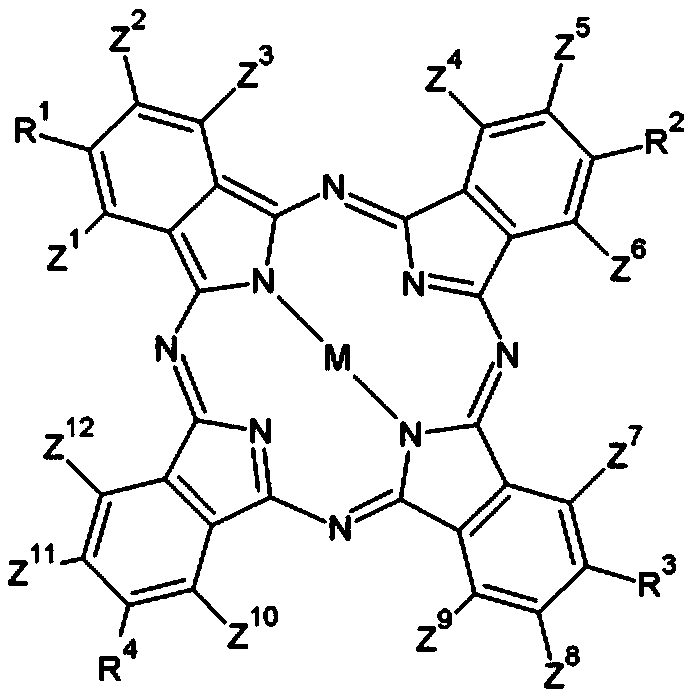
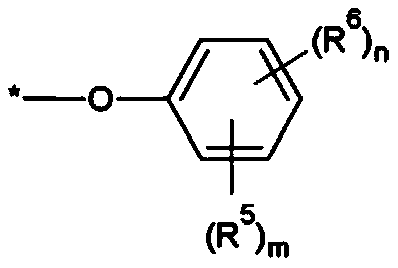
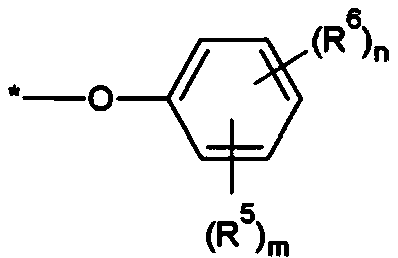
Compound, photosensitive resin composition including same, and color filter
A photosensitive resin and compound technology, applied in optical filters, zinc organic compounds, 2/12 group organic compounds without C-metal bonds, etc., can solve the problems of brightness and contrast ratio limitations, and achieve high molar extinction coefficient , good brightness and contrast ratio, the effect of improving the solubility of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0172] Synthesis Example 1: Synthesis of 3,5,6-trichloro-4-(2,4-di-tert-butylphenoxy)-phthalonitrile
[0173] 3,4,5,6-tetrachlorophthalonitrile (5g), 2,4-di-tert-butylphenol (3.8g), K 2 CO 3 (3.898 g) and N,N-dimethylformamide (50 ml) were placed in a 100 ml flask and then stirred while heating at 70°C. When the reaction was complete, the resultant was extracted with ethyl acetate (EA). After extraction, the product was purified by column chromatography with EA / hexane to obtain a liquid, the liquid was concentrated to obtain a solid, and the solid was dried in vacuo, thereby obtaining the compound represented by Synthesis Example 1.
Synthetic example 2
[0174] Synthesis Example 2: Synthesis of 3,5,6-trichloro-4-(2,6-dimethylphenoxy)-phthalonitrile
[0175] 3,4,5,6-tetrachlorophthalonitrile (5g), 2,6-dimethylphenol (6.6g), K 2 CO 3 (3.898g) and acetone (50ml) were placed in a 100ml flask and then stirred while heating at 50°C. When the reaction was complete, the resultant was extracted with EA (ethyl acetate). After extraction, the product was purified by column chromatography with EA / hexane to obtain a liquid, the liquid was concentrated to obtain a solid, and the solid was dried in vacuo, thereby obtaining the compound represented by Synthesis Example 2.
Synthetic example 3
[0176] Synthesis Example 3: Synthesis of 3,5,6-trichloro-4-(2-tert-butylphenoxy)-phthalonitrile
[0177] 3,4,5,6-tetrachlorophthalonitrile (5g), 2-tert-butylphenol (6.64g), K 2 CO 3 (3.898g) and acetone (50ml) were placed in a 100ml flask and then stirred while heating at 50°C. When the reaction was complete, the resultant was extracted with EA (ethyl acetate). After extraction, the product was purified by column chromatography with EA / hexane to obtain a liquid, the liquid was concentrated to obtain a solid, and the solid was dried in vacuo, thereby obtaining the compound represented by Synthesis Example 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com