Phthalocyanine compound, photosensitive resin composition, photosensitive resin layer, color filter and display device
A technology of compounds and phthalocyanines, applied in the field of display devices, can solve the problems of reduced pattern formability, reduced sensitivity, insufficient substrate adhesion, etc., and achieve excellent brightness and contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
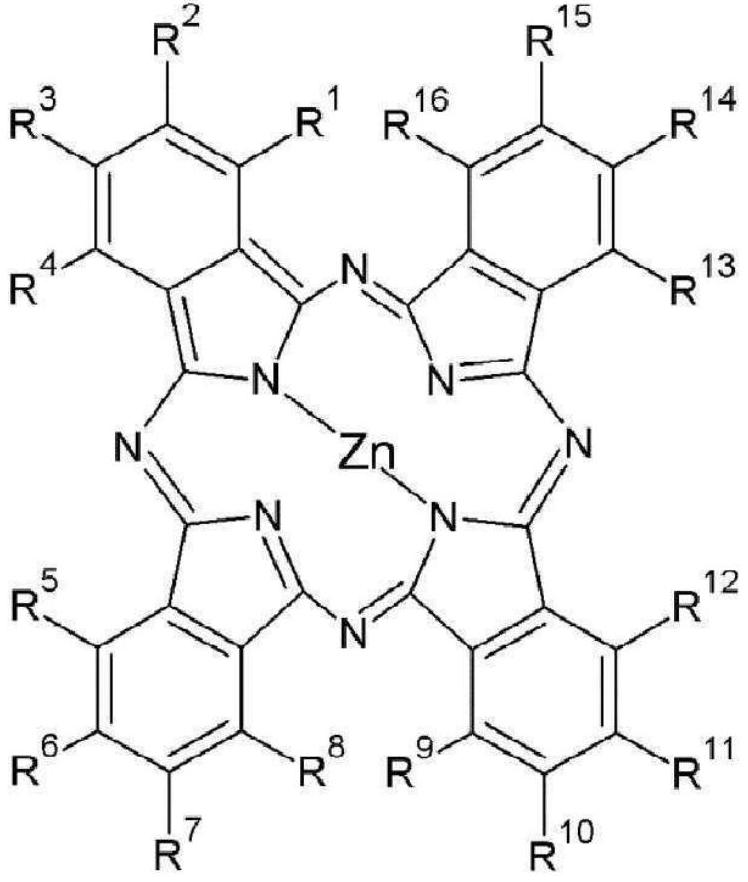
[0264] Synthesis Example 1: Synthesis of compounds represented by Chemical Formula 1-1
[0265] 3,4,5,6-Tetrachlorophthalonitrile (5 g), 2-isopropyl-phenol (2.56 g), K 2 CO 3 (3.9 g) and N,N-dimethylformamide (25 mL) were placed in a 100 mL flask and stirred while heating at 70°C. When the reaction was complete, ethyl acetate (EA) was used for extraction. After extraction, the resultant was concentrated and purified by column chromatography. After purification, the resultant was dried in vacuo to obtain 4-(2-isopropyl-phenoxy)-3,5,6-trichloro-phthalonitrile.
[0266] 4-(2-Isopropyl-phenoxy)-3,5,6-trichloro-phthalonitrile (2 g), 3,4,5,6-tetrachlorophthalonitrile ( 0.73 g), phthalonitrile (0.35 g), 1,8-diazabicycloundec-7-ene (2.08 g) and 1-pentenol (15 g) were placed in a 100 ml flask and Heat to 90°C to dissolve the solid, add zinc acetate (0.50 g), and stir the result while heating to 140°C. When the reaction was complete, methanol was used for precipitation, and the re...
Synthetic example 2
[0270] Synthesis Example 2: Synthesis of compounds represented by Chemical Formula 1-2
[0271] 4-(2-Isopropyl-phenoxy)-3,5,6-trichloro-phthalonitrile (2 g), 3,4,5,6-tetrachlorophthalonitrile ( 2.91 g), phthalonitrile (0.70 g), 1,8-diazabicycloundec-7-ene (4.16 g) and 1-pentenol (30 g) were placed in a 100 ml flask and Heat to 90°C to dissolve the solids, add zinc acetate (1.00 g), and stir the result while heating to 140°C. When the reaction was complete, methanol was used for precipitation, and the resulting precipitate was filtered and dried in vacuo. The dried solid was purified by column chromatography. Dichloromethane was appropriately added to the purified solid and dissolved, and methanol was added thereto to crystallize it. The crystalline solid was filtered and vacuum dried to obtain the compound represented by Chemical Formula 1-2.
[0272] [chemical formula 1-2]
[0273]
[0274] Maldi-tof MS:1090.96m / z
Synthetic example 3
[0275] Synthesis Example 3: Synthesis of compounds represented by Chemical Formula 1-3
[0276] 3,4,5,6-Tetrachlorophthalonitrile (5 g), 2-tert-butyl-phenol (2.83 g), K 2 CO 3 (3.9 g) and N,N-dimethylformamide (25 mL) were placed in a 100 mL flask and stirred while heating at 70°C. When the reaction was complete, ethyl acetate (EA) was used for extraction. After extraction, the resultant was concentrated and purified by column chromatography. After purification, the resultant was dried in vacuo to obtain 4-(2-tert-butyl-phenoxy)-3,5,6-trichloro-phthalonitrile.
[0277] 4-(2-tert-butyl-phenoxy)-3,5,6-trichloro-phthalonitrile (2 g), 3,4,5,6-tetrachlorophthalonitrile ( 0.70 g), phthalonitrile (0.34 g), 1,8-diazabicycloundec-7-ene (2.01 g) and 1-pentenol (15 g) were placed in a 100 ml flask and Heat to 90°C to dissolve the solid, add zinc acetate (0.48 g), and stir the result while heating to 140°C. When the reaction was complete, methanol was used for precipitation, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com