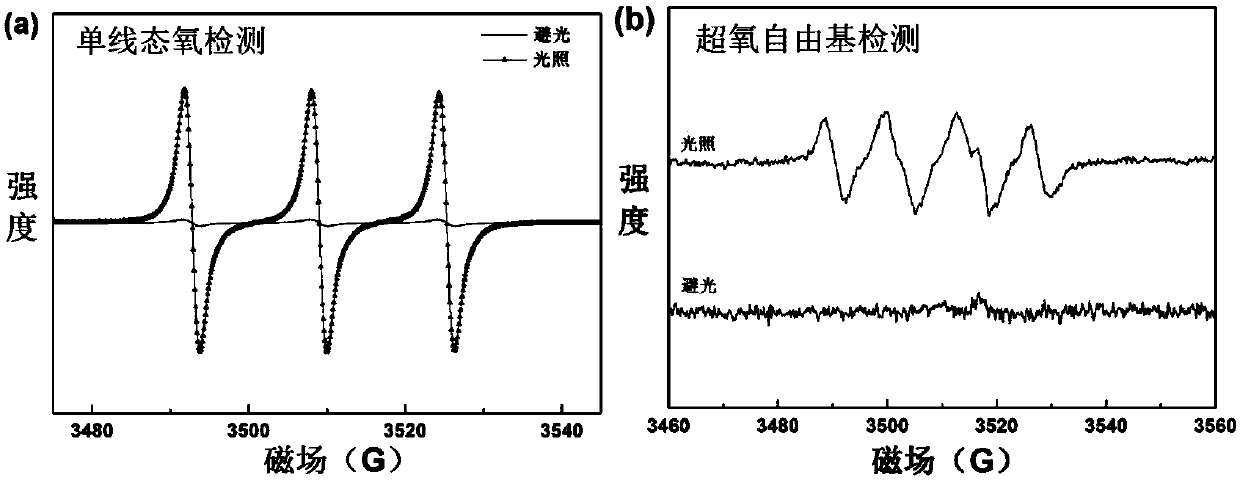
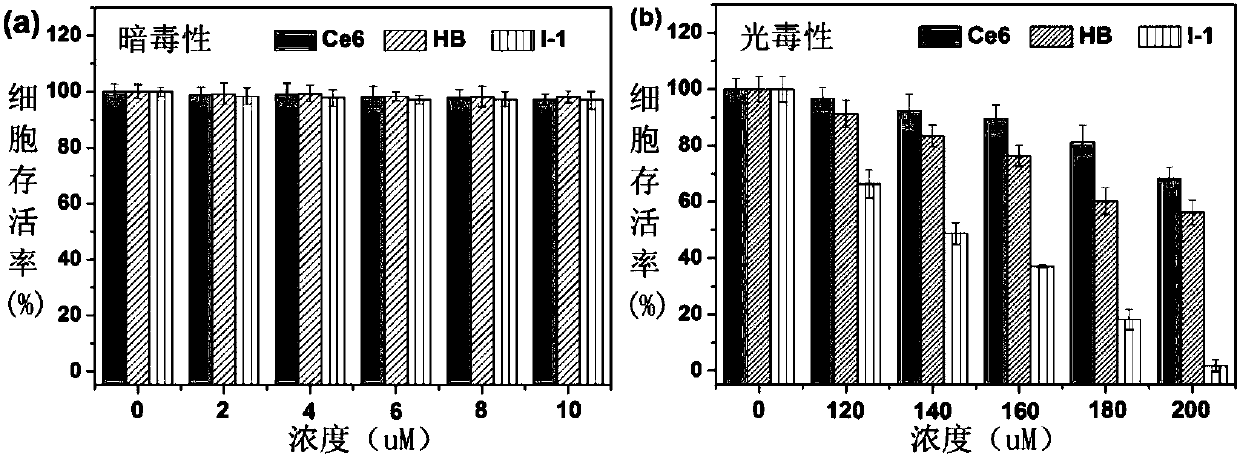
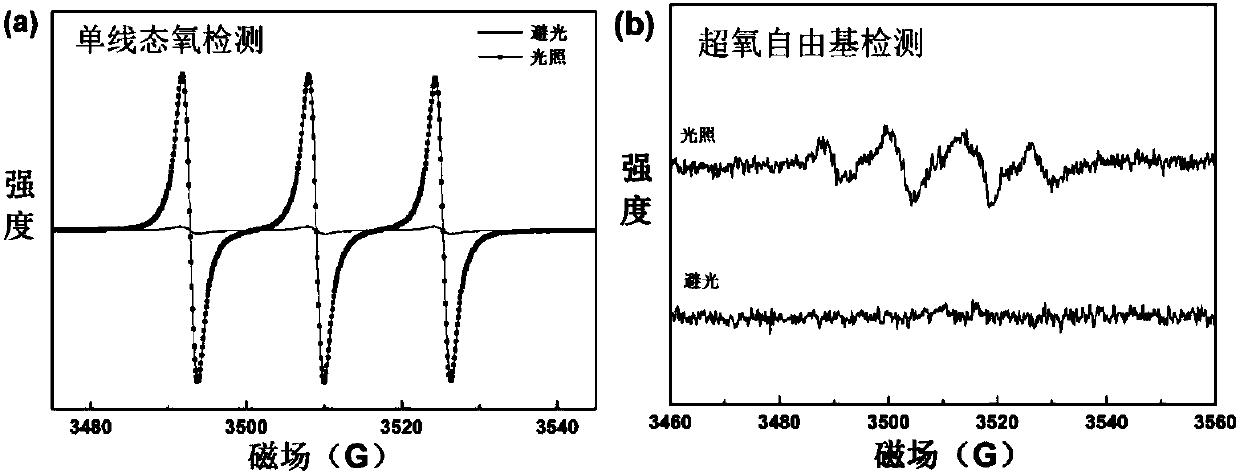
Poly-substituted near-infrared hypocrellin derivative, preparation method and applications thereof
A technology of hypocretin and its derivatives, which is applied in the field of multi-substituted near-infrared hypocretin derivatives and their preparation, and can solve the problems of increased molar extinction coefficient and red shift
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Hypocretin B (HB) and deacetylated hypocrellin B used in the present invention can be extracted and prepared by the following method, and can also be obtained through commercial purchase.
[0053] Extraction of Hypocretin A (HA): HA is extracted from natural Hypocrethin, reference (Organic Chemistry, 1989, 9, 252-254). 100g bamboo red fungus is pulverized with a pulverizer, placed in a Soxhlet extractor, continuously extracted for one day with 1000mL acetone as a solvent until the extract is nearly colorless, the extract is filtered to remove a small amount of insoluble solid insolubles, then spin-dried to remove acetone, and use 500mL dichloromethane was dissolved, washed with 4×400mL distilled water, the organic layer was separated and spin-dried, the solid residue was washed with 5×100mL petroleum ether, the solid was spontaneously ignited and dried in air, and then recrystallized twice with chloroform-petroleum ether to obtain The crystal is the target product HA, a...
Embodiment 2
[0057]
[0058] The preparation method of compound 1 refers to the literature: Photoreactions of hypocrellin B with thiolcompounds, Journal of Photochemistry and Photobiology B: Biology, 1998, 44, 45-52; Synthesis of a new water-soluble phototherapeutic sensitizer from hypocrellin B with enhanced red of absorption, Synthesis a new water-soluble phototherapeutic sensitizer, Dyes and Pigments, 1999, 4, 93-100. Put 10 groups of 5 mL of hypocretin B HB (0.2 mM) and mercaptoethylamine hydrochloride (0.01 mM) in ethanol / water buffer solution (1 / 3, pH=10) in 10 photochemical reactors at room temperature Illuminate with a 450W high-pressure sodium lamp for 20 minutes (filter the light below 470nm with a glass long-pass filter). After the reaction was completed, it was acidified with 10% hydrochloric acid, extracted with chloroform, and the chloroform phase was washed with water and spin-dried to obtain a crude product. The obtained crude product is further separated by silica gel ...
Embodiment 3
[0065]
[0066] The preparation method of compound 2 refers to the literature: Photoreactions of hypocrellin B with thiolcompounds, Journal of Photochemistry and Photobiology B: Biology, 1998, 44, 45-52; Synthesis of a new water-soluble phototherapeutic sensitizer, Dyes and Pigments, 1999, 4, 93 -100. Put 10 groups of 5mL hypocretin B HB (0.5mM) and cysteine hydrochloride (0.05mM) in methanol / water buffer solution (1 / 3, pH=11) into 10 photochemical reactors, Illuminate with a 450W high-pressure sodium lamp for 20 minutes at room temperature (filter light below 470nm with a glass long-pass filter). After the reaction was completed, it was acidified with 10% hydrochloric acid, extracted with chloroform, and the chloroform phase was washed with water and spin-dried to obtain a crude product. The obtained crude product was further separated by silica gel plate chromatography, and the developer was chloroform:methanol (volume ratio 98:1), to obtain 4 and 5 substituted, 8 and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com