Crystal form B of aspirin eugenol ester and preparation method thereof
A technology of eugenol ester and aspirin, applied in the field of medicine, can solve the problems such as the preparation method of aspirin eugenol ester crystal form that has not been disclosed by a patent, and achieve the effects of good stability, low cost and simple operation of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
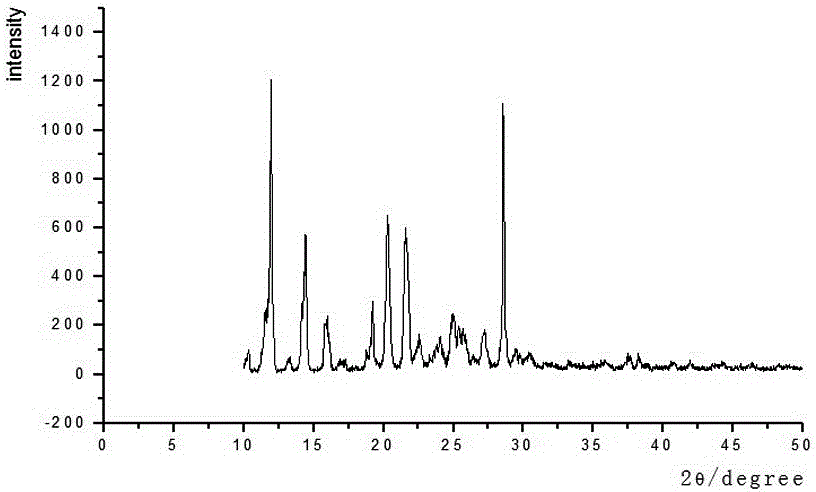
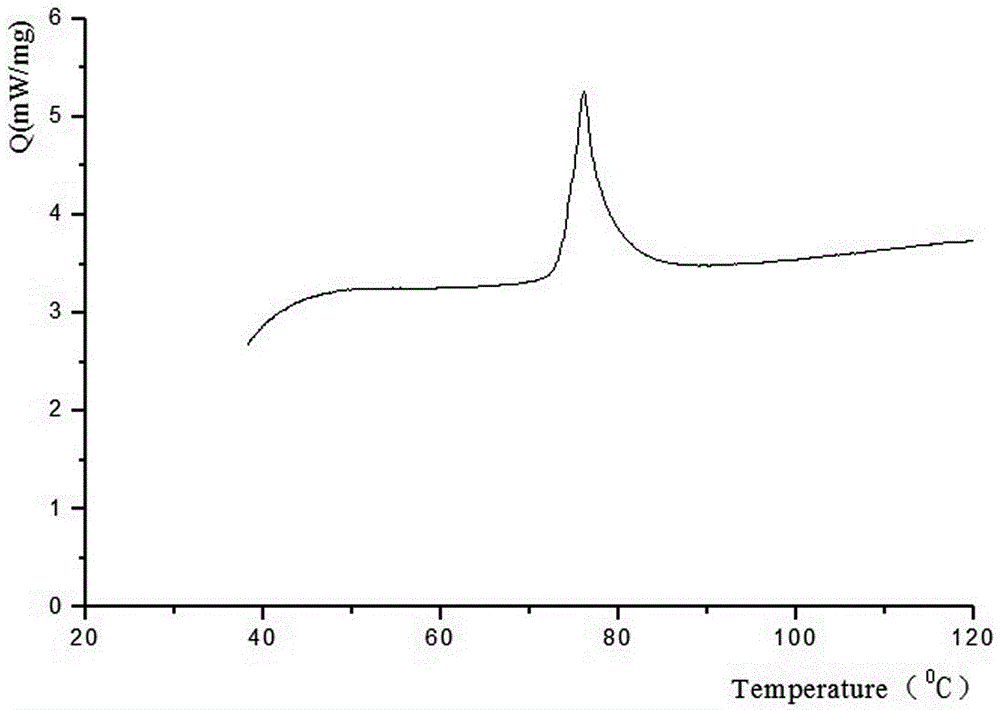
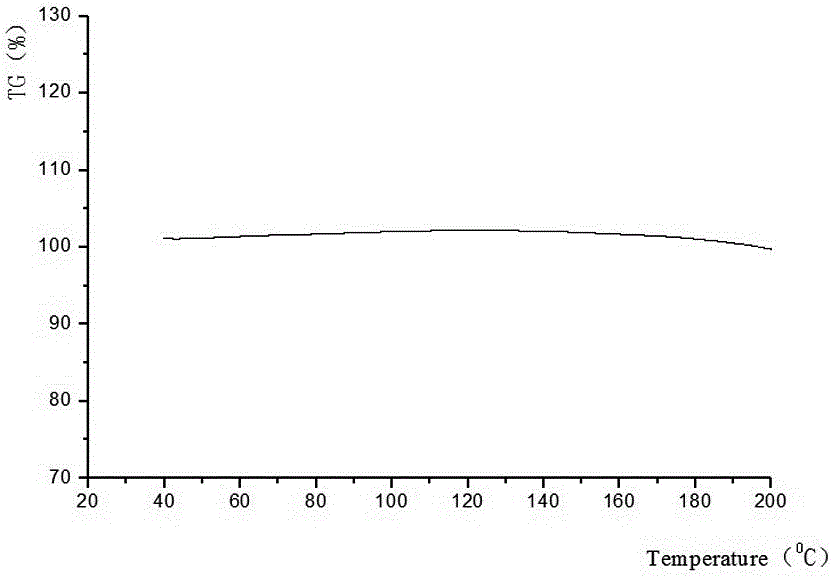
[0023] The invention discloses a method for preparing crystal form B of aspirin eugenol ester, which is prepared by adding an anti-solvent and hot-melting and cooling.
[0024] Anti-solvent addition method is used to prepare crystal form B, which is obtained by heating and dissolving solid aspirin eugenol ester in absolute ethanol, adding an appropriate amount of distilled water dropwise, and collecting the obtained solid.
[0025] Crystal form B is prepared by hot-melt cooling method, which is obtained by placing aspirin eugenol ester in an appropriate amount of 95% ethanol, heating to 60°C until completely dissolved, naturally cooling to room temperature, and filtering.
Embodiment 1
[0045] Example 1 Preparation of aspirin eugenol ester crystal form B by anti-solvent addition method:
[0046] Weigh 30 g of aspirin eugenol ester, add an appropriate amount of absolute ethanol (150 mL), heat and stir to 60°C, and dissolve it completely. Slowly add 15 mL of distilled water dropwise, cool naturally to room temperature, place at 4°C for 24 hours to precipitate all crystals, filter, and dry at 45°C for 24 hours to obtain 28.85 g of crystals with a yield of 96.18%. After testing, the obtained sample was aspirin eugenol ester crystal form B.
Embodiment 2
[0047] Example 2 Preparation of aspirin eugenol ester crystal form B by hot melting and cooling method:
[0048] Weigh 50 g of aspirin eugenol ester, add an appropriate amount of 95% ethanol (250 mL), heat and stir to 60°C, and completely dissolve. Naturally cooled to room temperature, placed at 6°C for 36 hours to precipitate all the crystals, filtered, and dried at 45°C for 24 hours to obtain 49.03 g of crystals with a yield of 98.06%. After testing, the obtained sample was aspirin eugenol ester crystal form B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com