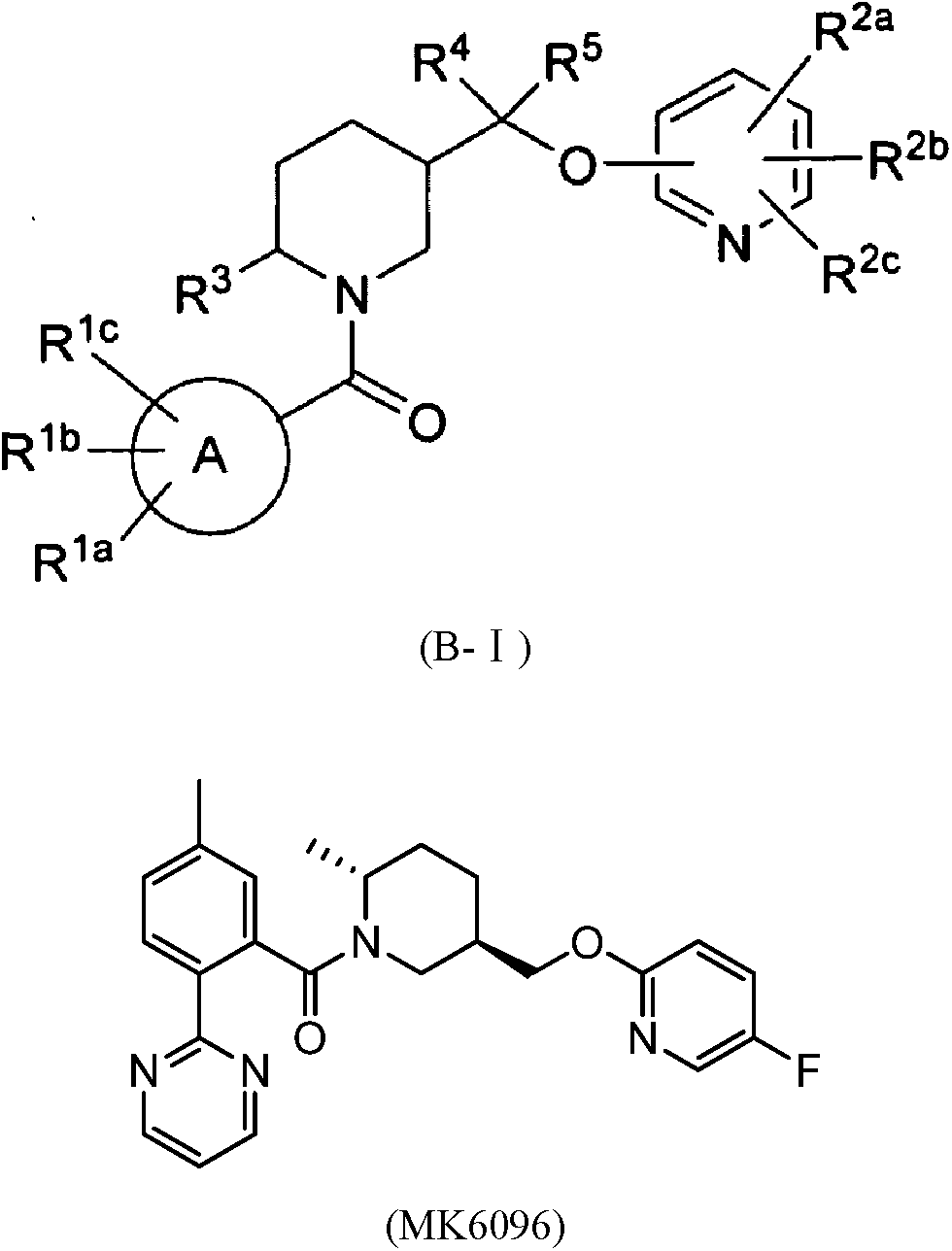
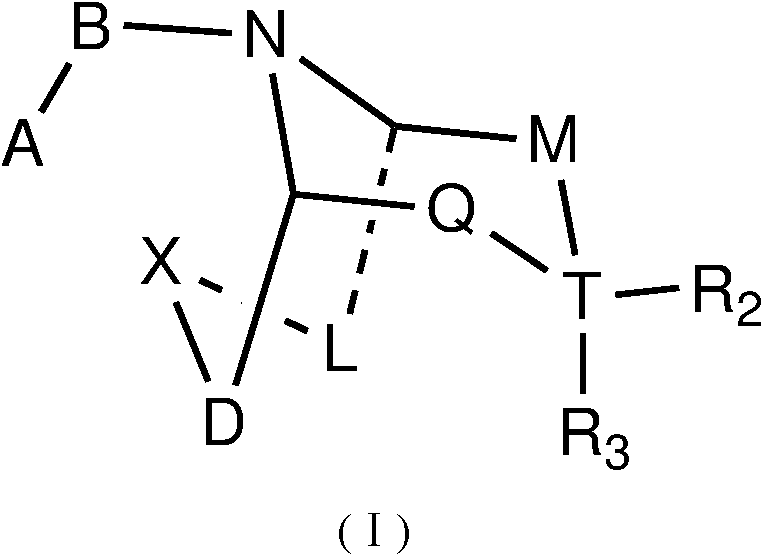
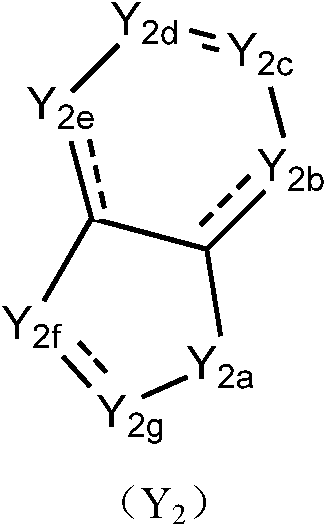
Piperidine derivatives as orexin receptor antagonists
A compound, selected technology, applied in the direction of drug combination, active ingredient of heterocyclic compounds, organic chemistry, etc., can solve problems such as solubility and pharmacokinetic half-life effect to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1,2
[0121]
[0122] A wavy bond indicates that the bond may face up or down and is not affected by other groups (the same below).
[0123] The first step (synthesis of 1-3)
[0124] Compound 1-1 (10.0 g, 44.4 mmol) was dissolved in 55 mL of tetrahydrofuran, LDA (24.4 mL, 0.0488 mol) was slowly added dropwise at -78°C, and stirred for 1 hour at -78°C. The temperature was kept at -78°C, and compound 1-2 was added dropwise to the reaction. After the addition, the temperature was slowly raised to room temperature, and the reaction was stirred overnight at room temperature. The reaction solution was poured into an aqueous ammonium chloride solution (50 mL), and concentrated under reduced pressure to obtain a crude product. Add 50mL saturated aqueous sodium chloride solution to it, extract with ethyl acetate (100mL×3), combine the organic phases, wash with water (100mL×2), saturated sodium chloride solution (100mL×2) successively, wash with anhydrous sodium sulfate Drying, filtrati...
Embodiment 3,4
[0151]
[0152]
[0153] The first step (synthesis of 3-1)
[0154] Compound 1-12 (100 mg) was dissolved in 4 mL of ethyl acetate, ethyl hydrogen chloride acetate (4 mL, 4M) was added dropwise under ice-bath conditions, stirred for 2 hours, and concentrated under reduced pressure to obtain product 3-1 (hydrochloride salt form) , the product was directly carried out to the next reaction without purification.
[0155] The second step (synthesis of 3-2)
[0156]Compound 3-1 (100mg, 0.26mmol), Compound 1-14 (58mg, 0.28mmol), HATU (150mg, 0.39mmol) and DIEA (124mg, 0.96mmol) were dissolved in 5mL of DMF and stirred at room temperature for 3 hours , the reaction solution was poured into saline solution and extracted with ethyl acetate (10mL×3), the organic phases were combined, washed with water (10mL×2) and saturated sodium chloride solution (10mL×2) successively, dried, filtered and concentrated to obtain Crude. The crude product was separated by preparative HPLC to obtai...
Embodiment 5,6
[0162]
[0163]
[0164] The first step (synthesis of 5-2)
[0165] Compound 1-12 (120mg, 0.32mmol), Compound 5-1 (77mg, 0.38mmol), HATU (182mg, 0.48mmol) and DIEA (124mg, 0.96mmol) were dissolved in 5mL of DMF and stirred at room temperature for 3 hours , the reaction solution was poured into saline solution and extracted with ethyl acetate (10mL×3), the organic phases were combined, washed with water (10mL×2) and saturated sodium chloride solution (10mL×2) successively, dried over anhydrous sodium sulfate, After filtration, the crude product was purified by preparative HPLC to obtain product 5-2 (24 mg, white solid, yield: 14%).
[0166] 1 H NMR (400MHz, METHANOL-d 4 )=8.83(br.s., 2H), 8.17-8.01(m, 2H), 7.49-7.33(m, 3H), 6.86(dd, J=3.5, 9.0Hz, 1H), 6.41(br.s. , 1H), 4.63 (br.s., 1H), 4.43 (br.s., 1H), 4.11 (br.s, 1H), 3.79 (br.s., 1H), 2.52-2.48 (m, 2H ), 2.35-2.11(m, 1H), 2.01-1.95(m, 3H), 1.90-1.67(m, 3H), 1.63-1.43(m, 1H), 1.29-1.20(m, 2H)
[0167] The second s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com