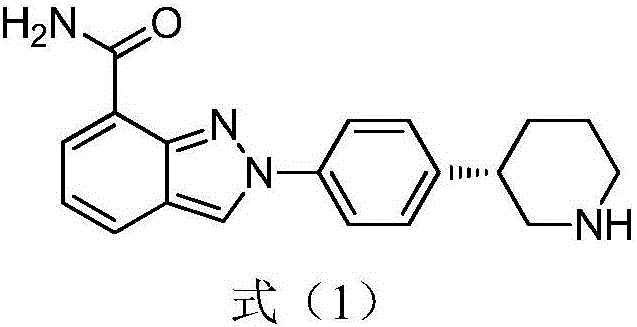
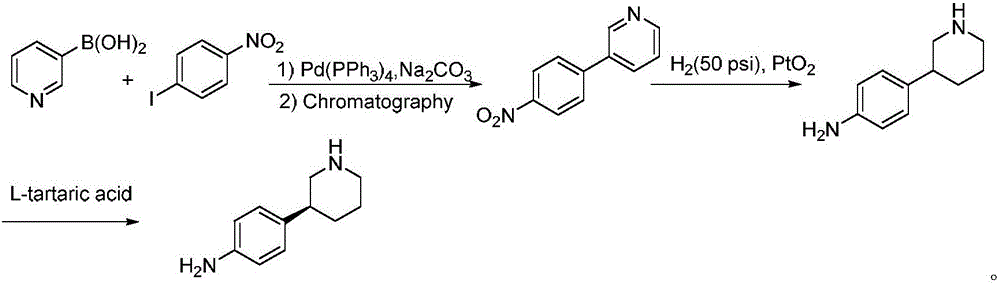
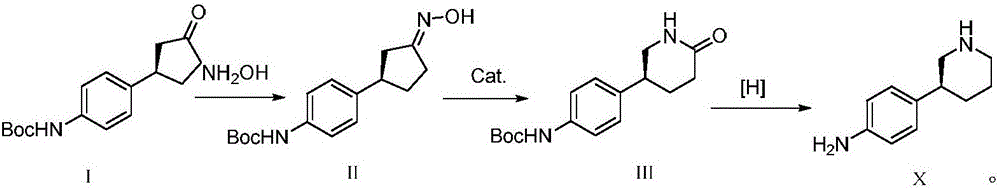
Preparation method of Niraparib intermediate 4-(3S-piperidine-3-yl)aniline
An intermediate, piperidine technology is applied in the field of preparation of anticancer drug Niraparib intermediate 4-aniline, which can solve the problems of high reaction cost, cumbersome processing, poor selectivity, etc., and achieves reduced production cost, fewer steps, and is conducive to scale. production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0031] The preparation of the compound shown in formula I
[0032] Add 0.35g (1mmol) of (S)-(-)-binaphthol phosphate to ethyl acetate, then add 2.32g (12mmol) of Boc-aniline and 0.82g (10mmol) of 2-cyclopentenone to the above In ethyl acetate at 10-20°C, the reaction time is 2-3 hours, concentrated, poured into water, then extracted with ether, concentrated, recrystallized from petroleum ether, and dried in vacuo to obtain 2.58 g of the compound represented by formula I, with a yield of 93.7%. The ee value is 99.21%, and the reaction process is as follows:
[0033]
preparation example 2
[0035] The preparation of the compound shown in formula I
[0036] Add 5.2g (15mmol) of (S)-(-)-binaphthol phosphate to ethyl acetate, then add 21.3g (110mmol) of Boc-aniline and 8.2g (100mmol) of 2-cyclopentenone to the above In ethyl acetate at 10-20°C, the reaction time is 2-3 hours, concentrated, poured into water, then extracted with ether, concentrated, recrystallized from petroleum ether, and vacuum-dried to obtain 25.9 g of the compound represented by formula I, with a yield of 94.2%. The ee value is 99.41%.
preparation example 3
[0038] The preparation of the compound shown in formula I
[0039] Add 0.17g (0.5mmol) of (S)-(-)-binaphthol phosphate to ethyl acetate, then add 2.13g (11mmol) of Boc-aniline and 0.82g (10mmol) of 2-cyclopentenone In the above ethyl acetate at 10-20°C, the reaction time is 2-3h, concentrated, poured into water, then extracted with ether, concentrated, recrystallized from petroleum ether, and vacuum-dried to obtain 2.55g of the compound represented by formula I, with a yield of 92.6% , ee value 99.17%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com