Tumorous specific antigen TSP70 and application thereof
A tumor antigen, tumor technology, applied in the direction of tumor-specific antigen, tumor rejection antigen precursor, anti-tumor drugs, etc., can solve the problem that the amino acid sequence of protein antigen cannot be directly inferred, and achieve high expression specificity and high expression positive rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Isolation and purification of TSP70 protein
[0030] 1. Preparation of human lung cancer cell line SPC-A1 cell lysate
[0031] 1) Cultivate SPC-A1 cells, discard the SPC-A1 cell culture medium, wash twice with Hanks solution, trypsinize for 2 minutes, add culture medium to resuspend the cells, and transfer the cell suspension to a 15mL (or 50mL) centrifuge tube , centrifuge at 1000rpm for 5 minutes, and discard the supernatant;
[0032] 2) Wash the cells once with pre-cooled PBS, centrifuge at 1000rpm for 5 minutes; transfer the cells to a 1.5mL EP tube, wash with PBS, and centrifuge at 12000pm for 5 minutes;
[0033] 3) Thaw the IP cell lysate on ice in advance. Take an appropriate amount of lysate (50uL / 10 6 cells), add 100mM protease inhibitors PMSF, PHI and PI in a ratio of 1:100;
[0034] 4) Add the lysate to the cell mass, and mix by pipetting;
[0035] 5) Incubate on ice for 30 minutes, vortex every 5 minutes;
[0036] 6) Centrifuge at 12000pm at ...
Embodiment 2
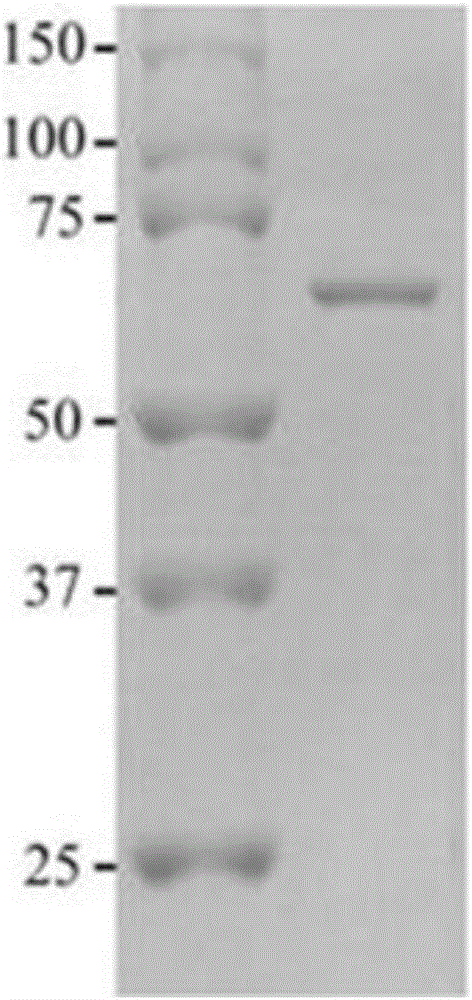
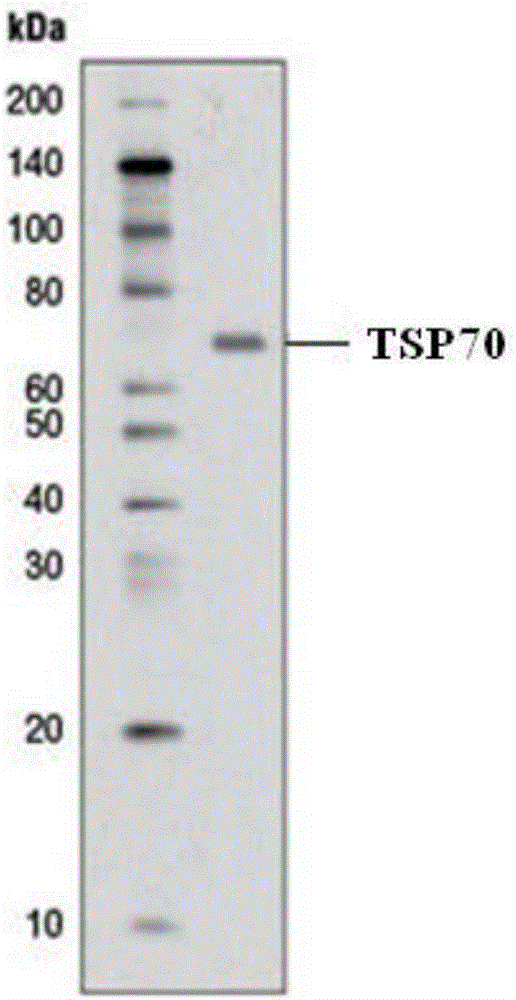
[0051] SDS-PAGE electrophoresis and Western blot identification of embodiment 2 TSP70 protein
[0052] 1 SDS-PAGE determination of protein molecular weight
[0053] The concentration of the separating gel is 12%, and the concentration of the stacking gel is 5%. After the sample is boiled for 3 minutes, the sample is added for electrophoresis, and the standard molecular weight is added as a control. The starting voltage is 80V, the sample is concentrated into a line and then increased to 120V after entering the separation gel, the total electrophoresis is about 2.5h, and Coomassie brilliant blue staining after stripping the gel. Electrophoresis results see figure 1 After electrophoresis, draw a molecular weight standard curve on semi-logarithmic coordinate paper according to the molecular weight of the standard protein and its relative mobility, and then find out that its molecular weight is 70Kda from the standard curve according to the relative mobility of the protein to be ...
Embodiment 3
[0056] Example 3 Determination of the amino acid sequence of the TSP70 protein
[0057] 1. Treat with 8mol / L urea to split the polypeptide chain.
[0058] 2. Determine the number of polypeptide chains by determining the relationship between the molar number of terminal amino acid residues and the molecular weight of the protein.
[0059] 3. In the presence of 8mol / L urea, treat with excess β-mercaptoethanol to break the disulfide bond and reduce it to sulfhydryl.
[0060] 4. Determine the amino acid composition of each polypeptide chain, and calculate the molecular ratio of the amino acid composition.
[0061] 5. Analysis of the N-terminal and C-terminal ends of the polypeptide chains.
[0062] 6. The polypeptide chain is broken into multiple peptides and separated.
[0063] 7. Determine the amino acid sequence of each peptide.
[0064] 8. Determine the order of the peptides in the polypeptide chain, and use the overlapping and overlapping of the amino acid sequences of tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com