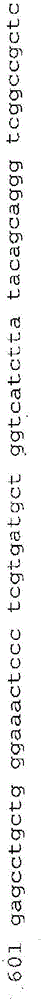Triaging of patients having asymptomatic hematuria using genotypic and phenotypic biomarkers
A gene expression, patient technology, applied in the direction of biochemical equipment and methods, biological testing, biological material analysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
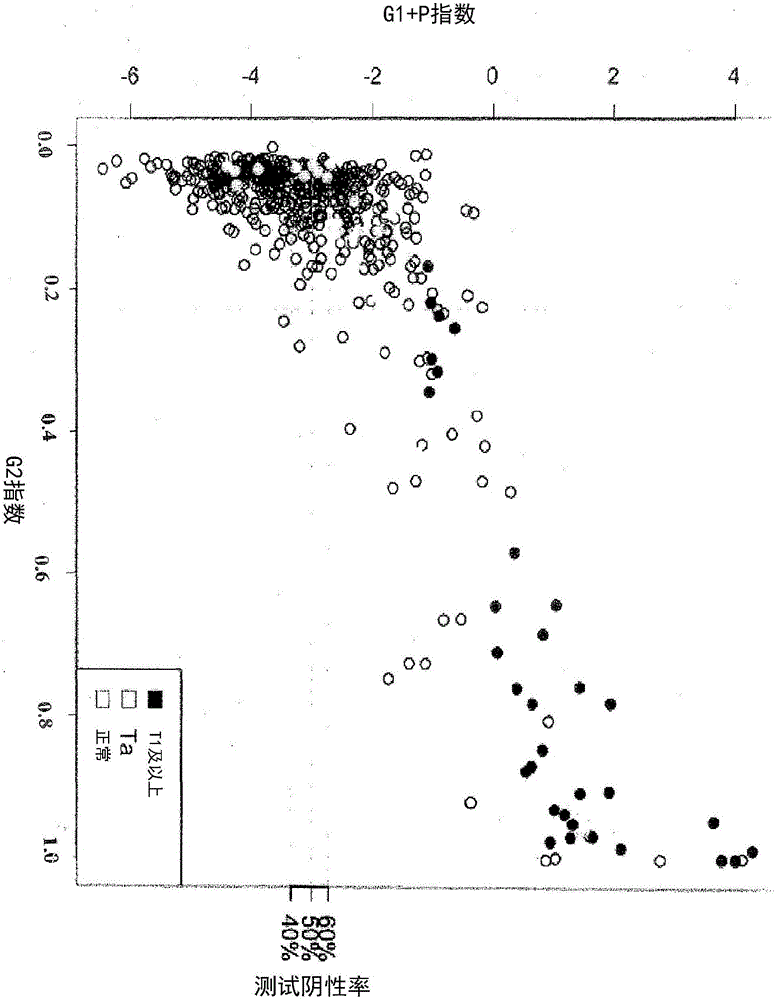
[0128] Thus, in certain preferred embodiments, a combination of genetic and phenotypic markers is provided that allows for bladder cancer from those at sufficient risk to warrant further clinical workup, possibly including cystoscopy or other The procedure identified patients with a low probability of having bladder cancer. In other embodiments, a marker is provided that has a reliability of greater than about 70%; in other embodiments, a reliability of greater than about 73%, and in still other embodiments, a reliability of greater than about 80%, In yet further embodiments, a reliability of greater than about 90%, in still other embodiments, a reliability of greater than about 95%, and in still further embodiments, a reliability of greater than about 98%, and in In some embodiments, about 100% reliability.
[0129] For genetic analysis, the practice of the present invention will employ, unless otherwise indicated, conventional techniques of molecular biology (including reco...
example 1
[0345] Example 1: Genotype Analysis of Bladder Cancer
[0346] method
[0347] Patients: Between April 2008 and September 2009, 485 patients with macroscopic hematuria but no history of urinary tract malignancy were recruited at 11 urology clinics in New Zealand and Australia. Each patient provided a urine sample immediately before undergoing cystoscopy and any additional diagnostic procedures. Three months into the study, a diagnosis was made. Of these 485 patients, gene expression data for all five studied genes were successfully obtained for 442 patients using the method described below. The characteristics of these patients are shown in Table 4.
[0348] Table 4: Characteristics of Study Population I
[0349]
[0350]
[0351] Table 4 shows the number of patients in each major diagnostic category at three months after their first overt hematuria.
[0352] Urinalysis: Urine samples were analyzed by Central Review Cytology (Southern Community Laboratories, Dunedin...
example 2
[0420] method
[0421] research group
[0422] A series of consecutive patients without a history of TCC were prospectively recruited between 28 April 2008 and 11 August 2009 at nine urology clinics in New Zealand and two urology clinics in Australia. The patient group included the patients used in Example 1, but also included an additional 46 patients whose data were not used in the first analysis. Further research also includes further analysis of the obtained results. Sample collection and RNA collection and testing were as described in Example 1.
[0423] RNA test development
[0424] Consists of four mRNA markers CDC2, HOXA13, MDK and IGFBP5. These markers were selected based on their low expression in blood and inflammatory cells and their overexpression in TCC. In this cohort study, we prospectively assigned a linear discriminant algorithm (uRNA-D), which combines four markers into a single score. uRNA-D is independent and was developed on a previous dataset. H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com