A kind of amorphous metal powder catalyst for decomposing water to produce hydrogen and preparation method thereof
A metal powder and catalyst technology, which is applied in the field of catalyst and its preparation, can solve the problems of high cost, poor stability, and high power consumption, and achieve the effects of uniform particle size, large specific area, and high hydrogen desorption activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
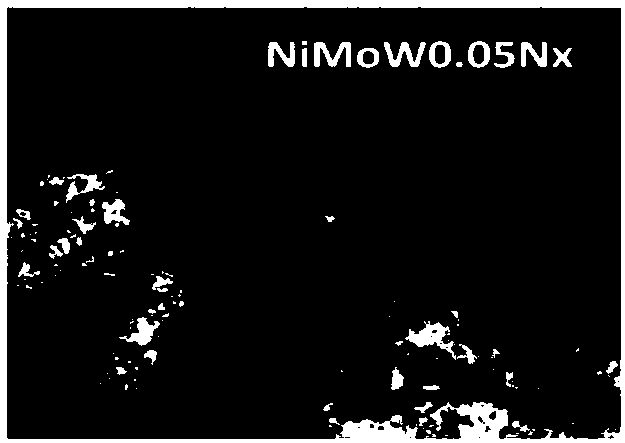
Embodiment 1
[0038] (1) Weigh 0.18880g of basic nickel carbonate (NiCO 3 2Ni(OH) 2 4H 2O), 14.781g of ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) quality, the ammonium metatungstate ((NH) of 0.3089g 4 ) 6 h 2 W 12 o 40 ·XH 2 O), it was put into a round bottom flask to obtain mixed powder. The molar ratio of each metal ion is 1:1:0.05.
[0039] (2) Measure 100 mL of deionized water, 30 mL of ethylene glycol, and 30 mL of triton, and mix the solutions evenly to obtain a mixed solution.
[0040] (3) Pour the measured mixed solution into a round bottom flask, mix the mixed powder evenly, put it in an oil bath at 150°C for reflux heating, and stir for 20 hours, then wash the reactant solution with deionized water To clear night to achieve colorless.
[0041] (4) Put the light green precipitate into an oven, heat it at 120° C. for 4 hours, then put it into a muffle furnace and bake it at 400° C. in air for 2 hours.
[0042] (5) Put a small amount of the catalyst precursor tha...
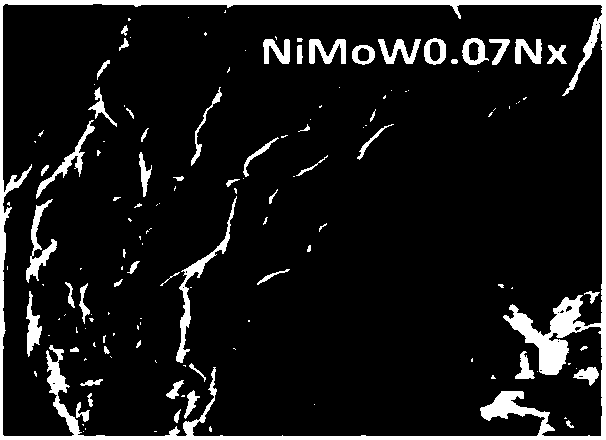
Embodiment 2
[0044] (1) Weigh 0.18880g of basic nickel carbonate (NiCO 3 2Ni(OH) 2 4H 2 O), 14.781g of ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O), 0.4324g of ammonium metatungstate ((NH 4 ) 6 h 2 W 12 o 40 ·XH 2 O), it is mixed and put into a round bottom flask to obtain mixed powder. The molar ratio of each metal ion is 1:1:0.07.
[0045] (2) Measure 100 mL of deionized water, 70 mL of ethylene glycol, and 70 mL of triton, and mix the solutions evenly to obtain a mixed solution.
[0046] The remaining steps are the same as (3)-(5) in Example 1, and the amorphous metal powder NiMoW for splitting water to produce hydrogen is finally obtained 0.07 N X . N X Indicates the Mo stored on the surface of the catalyst 2 The N content in the N part.
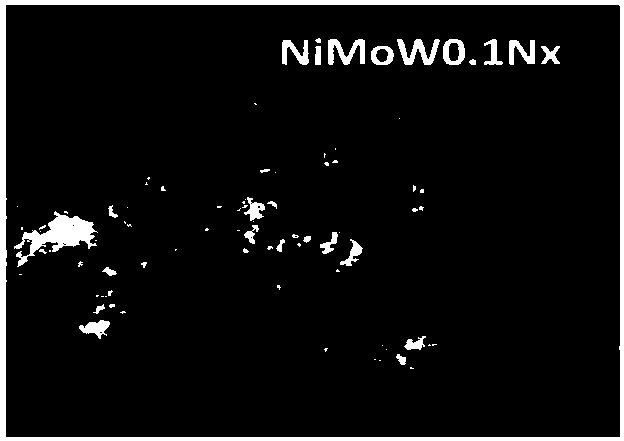
Embodiment 3
[0048] (1) Weigh 0.1880g of basic nickel carbonate (NiCO 3 2Ni(OH) 2 4H 2 O), 14.781g of ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O), 0.6179g of ammonium metatungstate ((NH 4 ) 6 h 2 W 12 o 40 ·XH 2 O), it is mixed and put into a round bottom flask to obtain mixed powder. The molar ratio of each metal ion is 1:1:0.1.
[0049] (2) Measure 100 mL of deionized water, 30 mL of ethylene glycol, and 70 mL of triton, and mix the solutions evenly to obtain a mixed solution.
[0050] (3) Pour the measured mixed solution into a round bottom flask, mix the mixed powder evenly, put it in an oil bath at 180°C for reflux heating, and stir for 25 hours, then wash the reactant solution with deionized water To clear night to achieve colorless.
[0051] (4) Put the light green precipitate into an oven, heat it at 150° C. for 3 hours, then put it into a muffle furnace and bake it at 300° C. in air for 4 hours.
[0052] (5) Put a small amount of the catalyst precursor that has...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com