Preparation method of sulfadoxine derivative
A kind of technology of sulfadoxine and derivatives, applied in the field of medicine and chemical industry, can solve the problems such as unreported synthesis and purification method of sulfadoxine derivative I
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 sulfadoxine derivative I
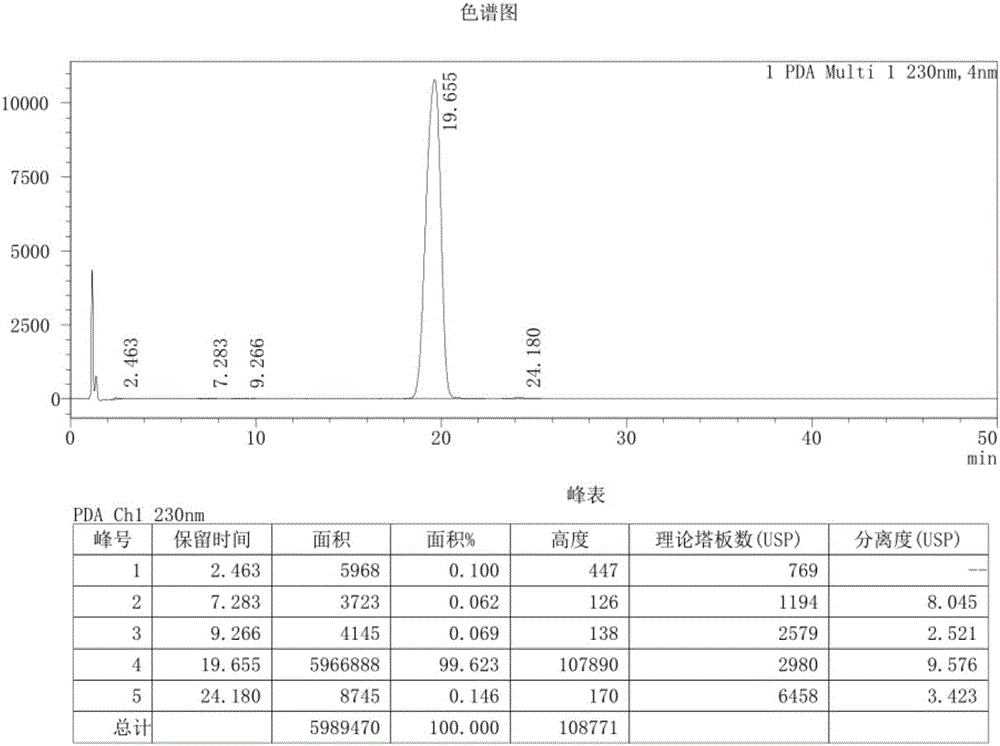
[0034] Add 20g of sulfadoxine and 100ml of acetic acid into the reaction bottle, start stirring, heat up to reflux for 8 hours, after the reaction, pour into 200ml of water, stir evenly, cool to below 10°C, add dropwise sodium carbonate solution to adjust pH=3- 5. Cool, filter with suction, wash, transfer the filter cake into a reaction flask, add 200ml of purified water, beat at room temperature for 1 hour, separate the solid by filtration, wash, and dry under reduced pressure at 60°C to obtain 10.3g of sulfadoxine derivative I. The HPLC detection purity is 99.62% (area normalization method), and the detection spectrum is as follows Figure 1 .
Embodiment 2
[0035] Embodiment 2 Preparation of sulfadoxine derivative I
[0036] Add 20g of sulfadoxine and 100ml of acetic anhydride into the reaction bottle, start stirring, heat up to reflux for 8 hours, after the reaction, pour into 200ml of water, stir evenly, cool to below 10°C, add dropwise sodium carbonate solution to adjust pH=3 -5, cooling, suction filtration, washing, transfer the filter cake into a reaction flask, add 200ml of purified water, beat at room temperature for 1 hour, filter and separate the solid, wash, and dry under reduced pressure at 60°C to obtain 13.8g of sulfadoxine derivative I. The purity detected by HPLC was 99.52% (area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com