A kind of preparation method of 6-phosphoryl substituted phenanthridine derivatives
A technology of derivatives and phosphoryl groups, which is applied in the field of preparation of 6-phosphorylphenanthridine derivatives, can solve the problems of complex post-processing, waste of raw materials, harsh reaction conditions, etc., achieve scientific and reasonable synthesis methods, reduce production costs, The effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
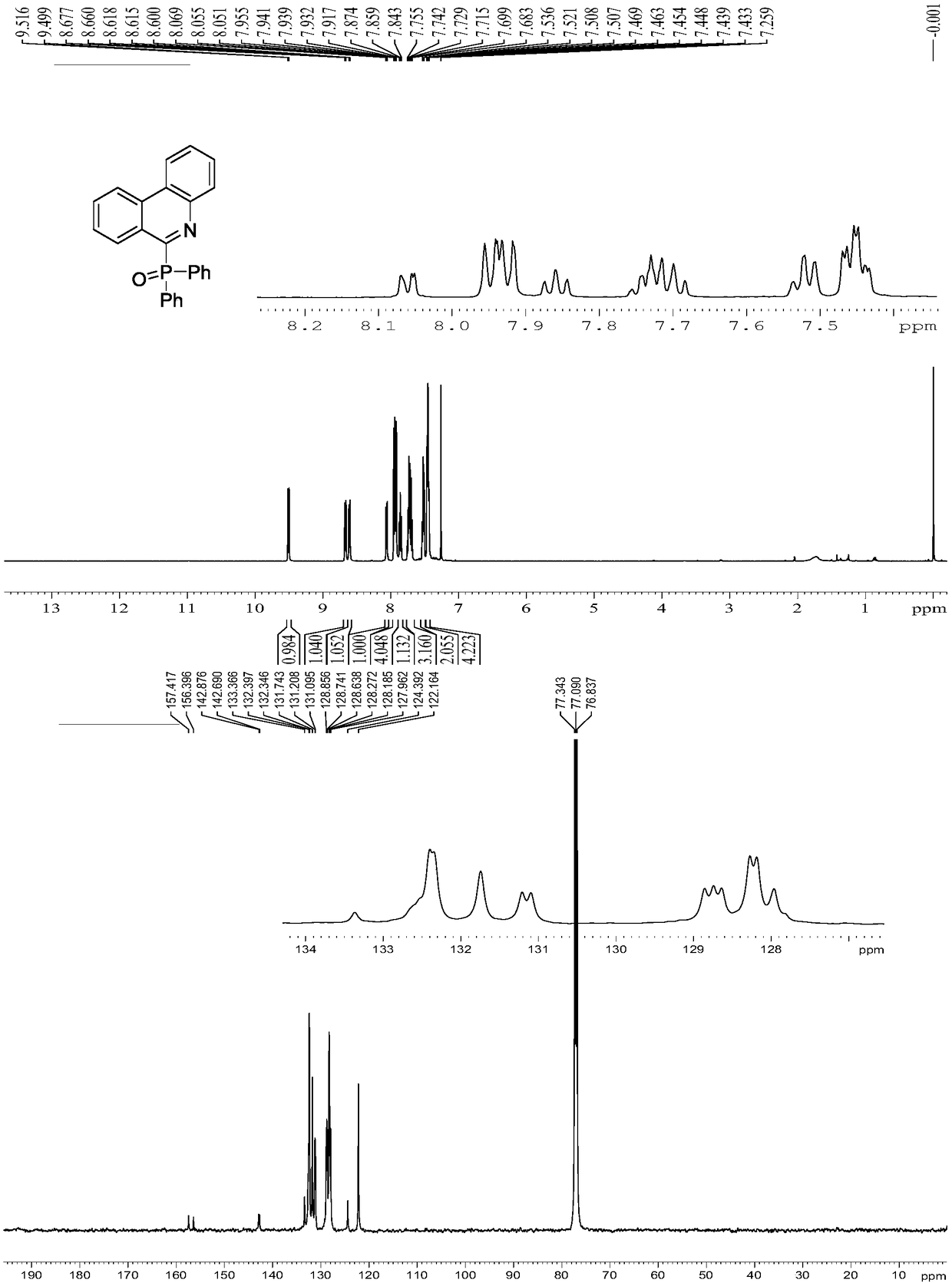
[0021] Preparation of 6-phosphorylphenanthridine 3a
[0022]
[0023] To a 10ml thick-walled pressure-resistant tube, add biphenylisothiocyanate 1a (0.4mmol, 84.4mg), diarylphosphine 2a (0.6mmol, 121.2mg), manganese acetate tetrahydrate (0.4mmol, 98.1mg), N,N-dimethylformamide (2ml) was added, after the addition was complete, the reaction was carried out at 110°C for 6.0h. After the reaction system was cooled, 10 ml of ethyl acetate was added to dilute the reaction system, and washed with 30 ml of saturated sodium chloride solution six times, the organic phases were combined, dried by adding magnesium sulfate for 30 minutes, filtered, and the filtrate was concentrated by rotary evaporation to obtain a crude product. The crude product was separated by column (200-300 mesh silica gel) with eluent (petroleum ether: ethyl acetate=2:1), and the white solid product was confirmed to be 6-phosphorylphenanthridine 3a by NMR, and the yield was 68 %.
[0024] Spectrum analysis data ...
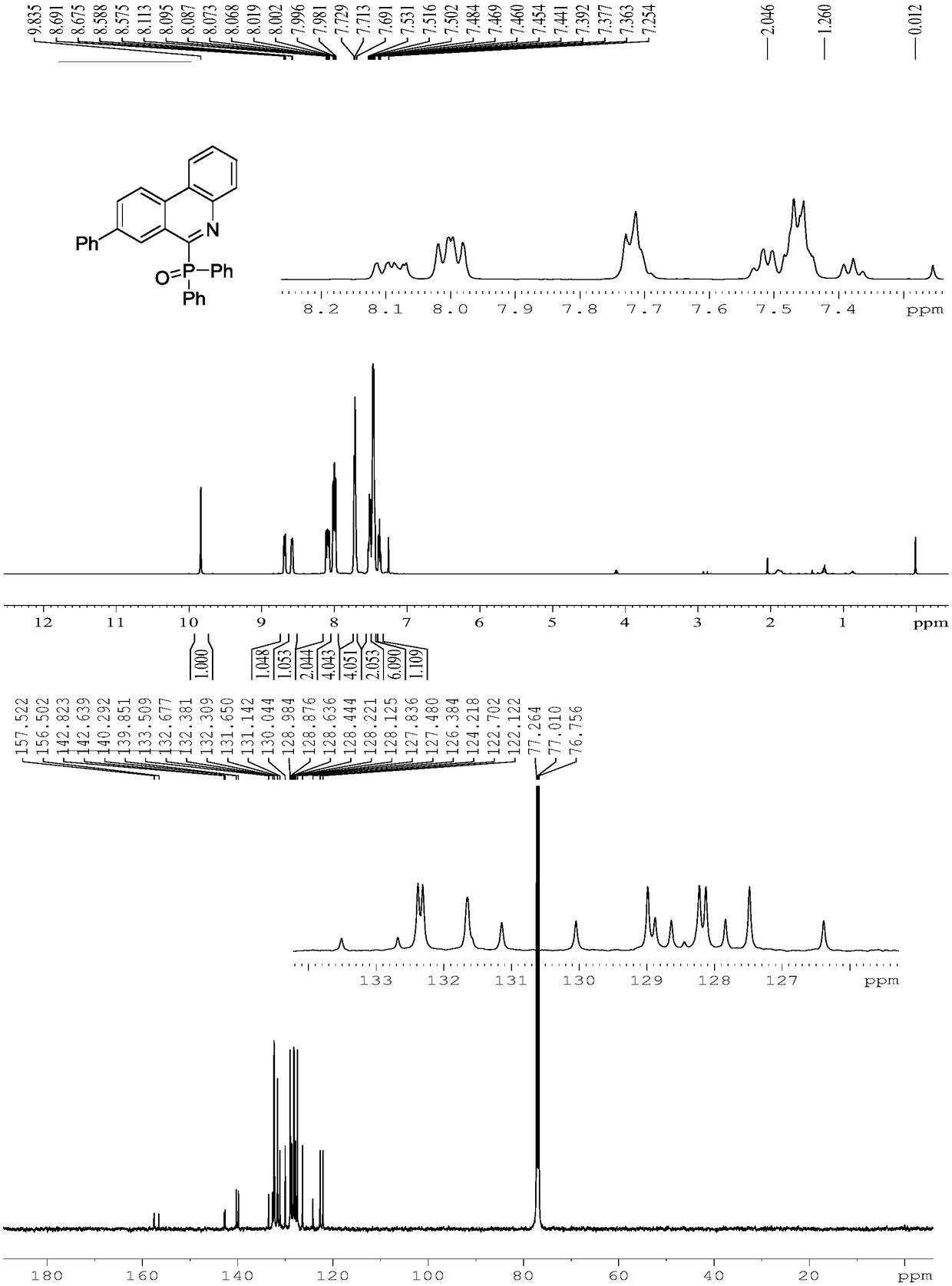
Embodiment 2
[0027] Replace 1a in Example 1 with 1b, and other experimental conditions are the same as Example 1, and the experimental results are shown in Table 1.
[0028]
[0029] Spectrum analysis data 3b:
[0030] 1 H NMR (CDCl 3 ,500MHz):δ2.56(s,3H),7.42-7.45(m,4H),7.51(t,J=7.3Hz,2H),7.65-7.72(m,3H),7.94(q,J=6.3 Hz,4H),8.03(d,J=7.9Hz,1H),8.54(d,J=3.7Hz,2H),9.33(s,1H). 13 C NMR (CDCl 3 ,125MHz): δ21.9,121.9,124.4,127.7,128.1(d,J=12.0Hz),128.7,130.5,131.0,131.6,132.3(d,J=8.0Hz),132.8(d,J=16.4Hz), 133.6, 138.1, 142.4(d, J=23.9Hz), 156.3(d, J=128.7Hz).
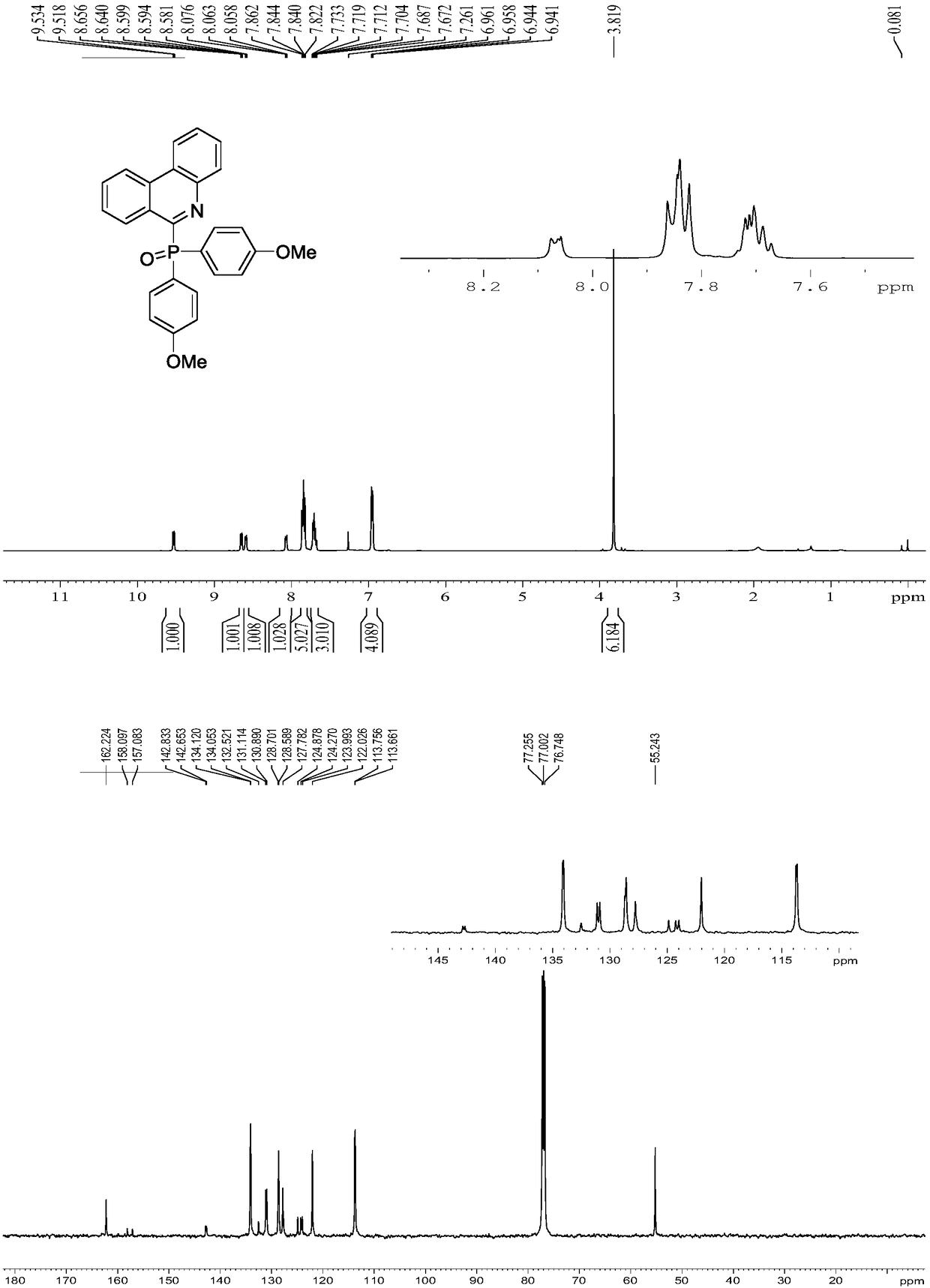
Embodiment 3
[0032] Replace 1a in Example 1 with 1c, and other experimental conditions are the same as Example 1, and the experimental results are shown in Table 1.
[0033]
[0034] Spectral analysis data 3c:
[0035] 1 H NMR (CDCl 3 ,500MHz):δ3.94(s,3H),7.43-7.48(m,5H),7.64(t,J=7.4Hz,2H),7.69(t,J=7.6Hz,2H),7.97(q, J=6.3Hz, 4H), 8.04(d, J=8.1Hz, 1H), 8.50(d, J=7.9Hz, 1H), 8.54(d, J=9.1Hz, 1H), 9.03(d, J= 2.2Hz,1H). 13 C NMR (CDCl 3 ,125MHz):δ55.6,107.5,121.6,122.6,123.6,124.5,127.1,127.6,128.1(d,J=11.4Hz),128.8,129.4(d,J=22.9Hz),131.1,131.6,132.3(d, J=7.9Hz), 132.6, 133.5, 142.1(d, J=22.8Hz), 155.5(d, J=129.6Hz), 158.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com