Luminous material, preparation method of luminous material and white light LED (light emitting diode) device
A technology for LED devices and luminescent materials, applied in the directions of luminescent materials, chemical instruments and methods, electrical components, etc., can solve the inconsistency of cross-absorption energy loss and thermal quenching behavior, unfavorable commercialization and popularization of white LEDs, and increasing types of white light LED manufacturing cost and other issues, to achieve the effect of reducing self-absorption energy loss, low price, and reducing self-absorption energy loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
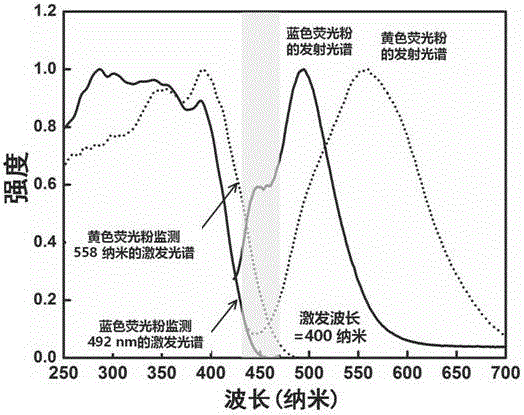
[0032] This embodiment provides a luminescent material, including blue phosphor and yellow phosphor.
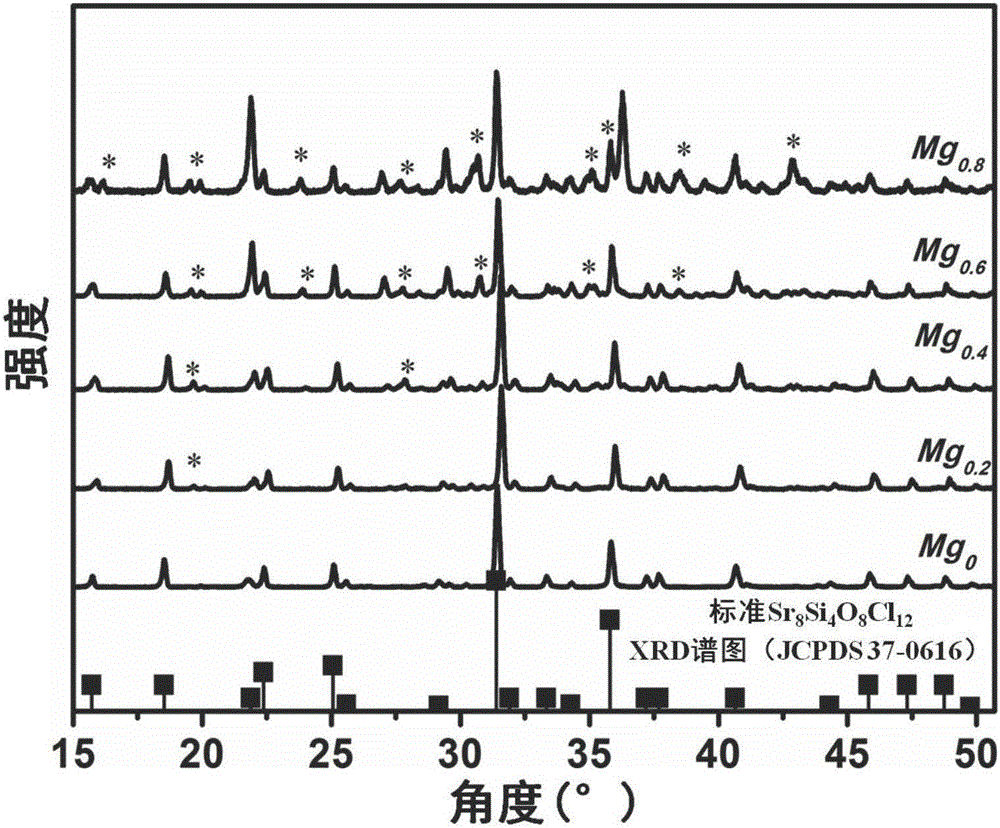
[0033] 1-1. Blue phosphor preparation method The chemical composition formula of blue phosphor is (Sr 8-x-z Mg x ) Si 4 o 8 Cl 12 :zEu 2 + , where x=0.2, z=0.05, the specific expression (Sr 7.75 Mg 0.2 ) Si 4 o 8 Cl 12 :0.05Eu 2+ .
[0034] ① Weigh the following materials:
[0035] SrCO 3 : 2.768g, SrCl 2 ·6H 2 O: 5.332g, SiO 2 : 1.204g, Eu 2 o 3 : 0.04399g, MgO: 0.0403g
[0036] ② After fully grinding the above-mentioned weighed materials for 15 minutes, put the mixed powder into an alumina crucible or a carbon crucible, and put it into a high-temperature furnace under 10% H 2 / 90%N 2 Anneal at a high temperature of 1000 C for 3 hours in a reducing atmosphere, cool, take out and grind finely to obtain a Excited powder crystals of blue phosphors with dual wavelength emission.
[0037] 1-2. Preparation method of yellow fluorescent powder The chemical co...
Embodiment 2
[0042] This embodiment provides a luminescent material, including blue phosphor and yellow phosphor.
[0043] 2-1. Preparation method of blue phosphor
[0044] The chemical composition formula of the blue phosphor is (Sr 8-x-z Mg x ) Si 4 o 8 Cl 12 :zEu 2+ , where x=0.6, z=0.05, the specific expression (Sr 7.35 Mg 0.6 ) Si 4 o 8 Cl 12 :0.05Eu 2+ .
[0045] ① Weigh the following materials:
[0046] SrCO 3 : 2.742g, SrCl 2 ·6H 2 O: 5.3324g, SiO 2 : 1.204g, Eu 2 o 3 : 0.04399g, MgO: 0.1209g;
[0047] ② Grind the above-mentioned weighed materials thoroughly for 20 minutes, put the mixed powder into an alumina crucible or carbon crucible, put it in a high-temperature furnace and anneal at 1000 C for 3 hours under a CO reducing atmosphere, cool it, take it out and grind it into fine powder. get fit in violet Excited powder crystals of blue phosphors with dual wavelength emission.
[0048] 2-2. Preparation method of yellow phosphor The chemical composition form...
Embodiment 3
[0053] This embodiment provides a luminescent material, including blue phosphor and yellow phosphor.
[0054] 3-1. Preparation method of blue phosphor
[0055] The chemical composition formula of the blue phosphor is (Sr 8-x-z Mg x ) Si 4 o 8 Cl 12 :zEu 2+ , where x=0.8, z=0.05, the concrete expression (Sr 7.15 Mg 0.8 ) Si 4 o 8 Cl 12 :0.05Eu 2+ .
[0056] ① Weigh the following materials:
[0057] SrCO 3 : 2.325g, SrCl 2 ·6H 2 O: 5.3324g, SiO 2 : 1.204g, Eu 2 o 3 : 0.04399g, MgO: 0.1612g;
[0058] ② After fully grinding the above-mentioned weighed materials for 30 minutes, put the mixed powder into an alumina crucible or a carbon crucible, and put it into a high-temperature furnace under 10% H 2 / 90%N 2 Anneal at a high temperature of 1000 C for 3 hours in a reducing atmosphere, cool, take out and grind finely to obtain a Excited powder crystals of blue phosphors with dual wavelength emission.
[0059] 3-2. Preparation method of yellow phosphor
[0060...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com