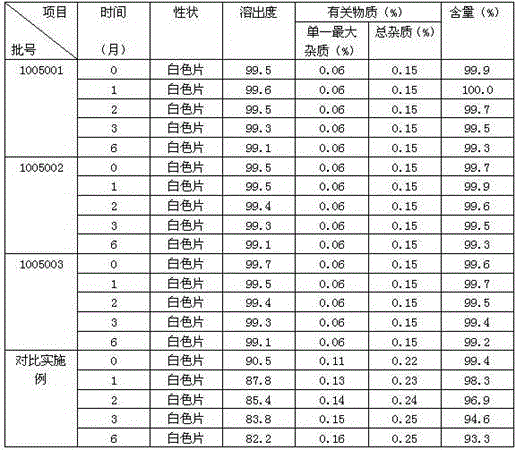
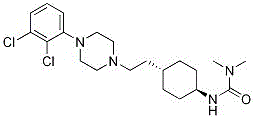
Cariprazine pharmaceutical composition and preparation method thereof
The technology of cariprazine and composition is applied in the field of cariprazine pharmaceutical composition and preparation thereof, which can solve the problems of difficult purification of cariprazine, patent protection of cariprazine, high impurity content, and high bioavailability. The effect of stability, product quality and fast dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The described cariprazine pharmaceutical composition of every 1000, its formula consists of:
[0049] Cariprazine 5g
[0050] Hydroxypropyl Cellulose 20g
[0051] Lactose 75g
[0052] Microcrystalline Cellulose 30g
[0054] Preparation Process:
[0055] 1) Preparation: dry microcrystalline cellulose, lactose, and magnesium stearate at 80°C for 4 hours, and set aside
[0056] 2) Put the prescribed amount of hydroxypropyl cellulose and cariprazine in a container, mix well, grind until the particle size is 80±10um, and set aside;
[0057] 3) Mix item 2) with the prescribed amount of microcrystalline cellulose and magnesium stearate evenly;
[0058] 4) Tablet compression: adjust the appropriate hardness and tablet weight, and perform tablet compression;
[0059] 5) Packing: packed by aluminum-plastic blister packing machine;
[0060] 6) Storage.
Embodiment 2
[0062] The described cariprazine pharmaceutical composition of every 1000, its formula consists of:
[0063] Cariprazine 3.5g
[0064] Hydroxypropyl Cellulose 14g
[0065] Lactose 52.5g
[0066] Microcrystalline Cellulose 45g
[0068] Preparation process: with embodiment 1.
Embodiment 3
[0070] The described cariprazine pharmaceutical composition of every 1000, its formula consists of:
[0071] Cariprazine 12g
[0072] Hydroxypropyl Cellulose 48g
[0073] Lactose 180g
[0074] Microcrystalline Cellulose 72g
[0076] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com