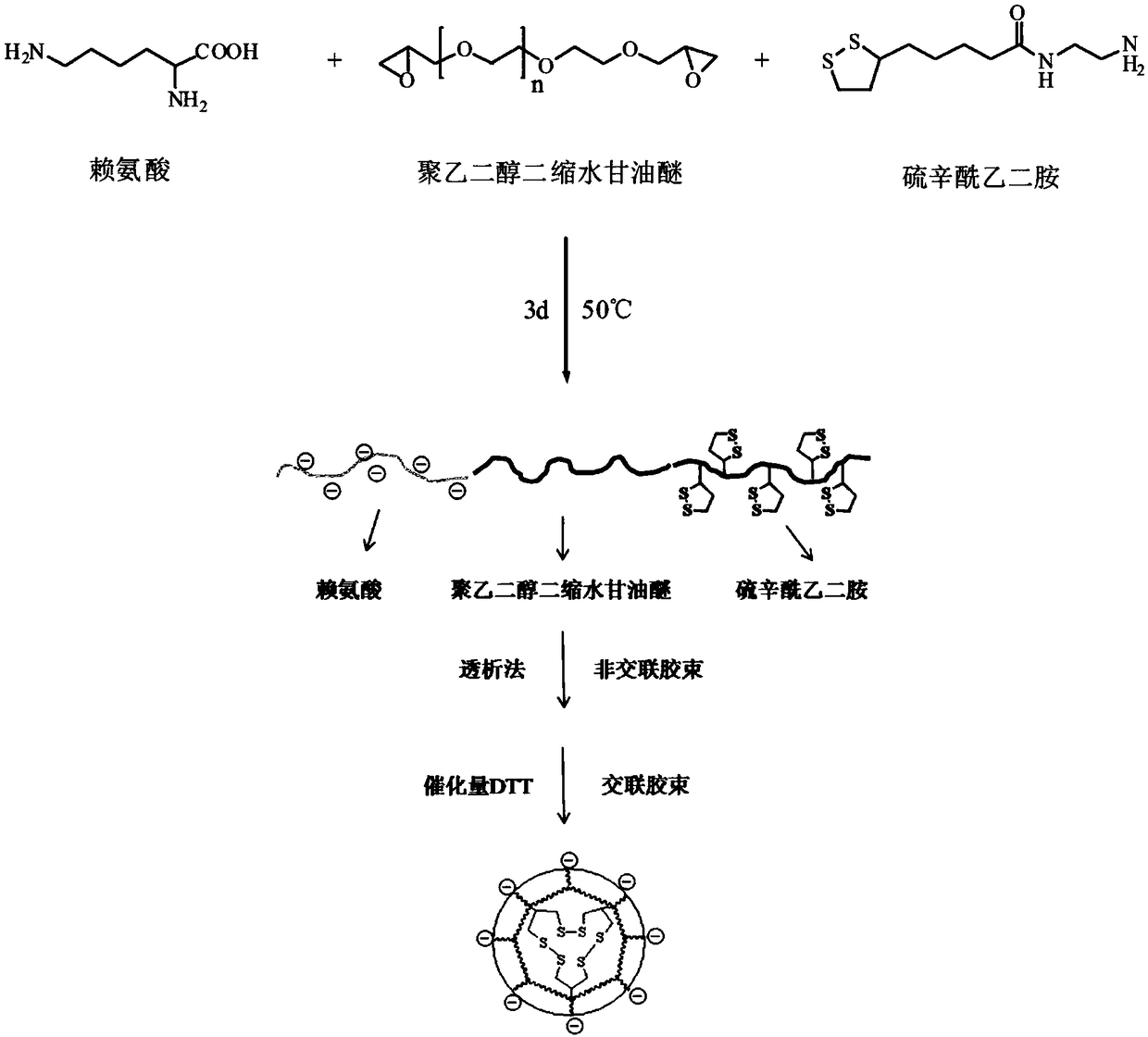
A kind of preparation method of charge-reversible reduction-sensitive reversible cross-linked nanomicelles
A nanomicelle and charge technology, applied in the field of preparation of reduction-sensitive reversible cross-linked nanomicelles, can solve the problems of reducing the application feasibility of such materials, cumbersome reactions, and the inability of materials to meet biodegradability, and achieve excellent resistance. Protein adsorption performance, mild and efficient reaction, and the effect of increasing the difficulty of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1) Synthesis of lipoyl ethylenediamine:
[0047] Weigh 3.00g (1.45×10 -2 mol) lipoic acid and 2.59g (1.60×10 -2 mol) N,N'-carbonyldiimidazole, dissolved in 30mL of chloroform, reacted for 1 hour at 25°C under nitrogen protection, then transferred the mixture to the dropping funnel, and added dropwise evenly stirred ethylenediamine (8mL , 0.12mol) in chloroform (30mL) solution, reacted for 12 hours at 25°C under nitrogen protection. After the reaction was completed, the reaction mixture was transferred to a separatory funnel, extracted with 10% NaCl (100mL) and 1M NaOH (100mL) respectively, and the organic phase was collected, and the organic solvent was removed by rotary evaporation to obtain a yellow gel-like compound Thioct Ethylenediamide (2.2 g, 61%).
[0048] 2) Synthesis of poly(lysine-co-polyethylene glycol diglycidyl ether-co-lipoyl ethylenediamine):
[0049] Weigh 315mg (1mmol) polyethylene glycol diglycidyl ether, 102mg (0.7mmol) lysine, and 75mg (0.3mmol)...
Embodiment 2
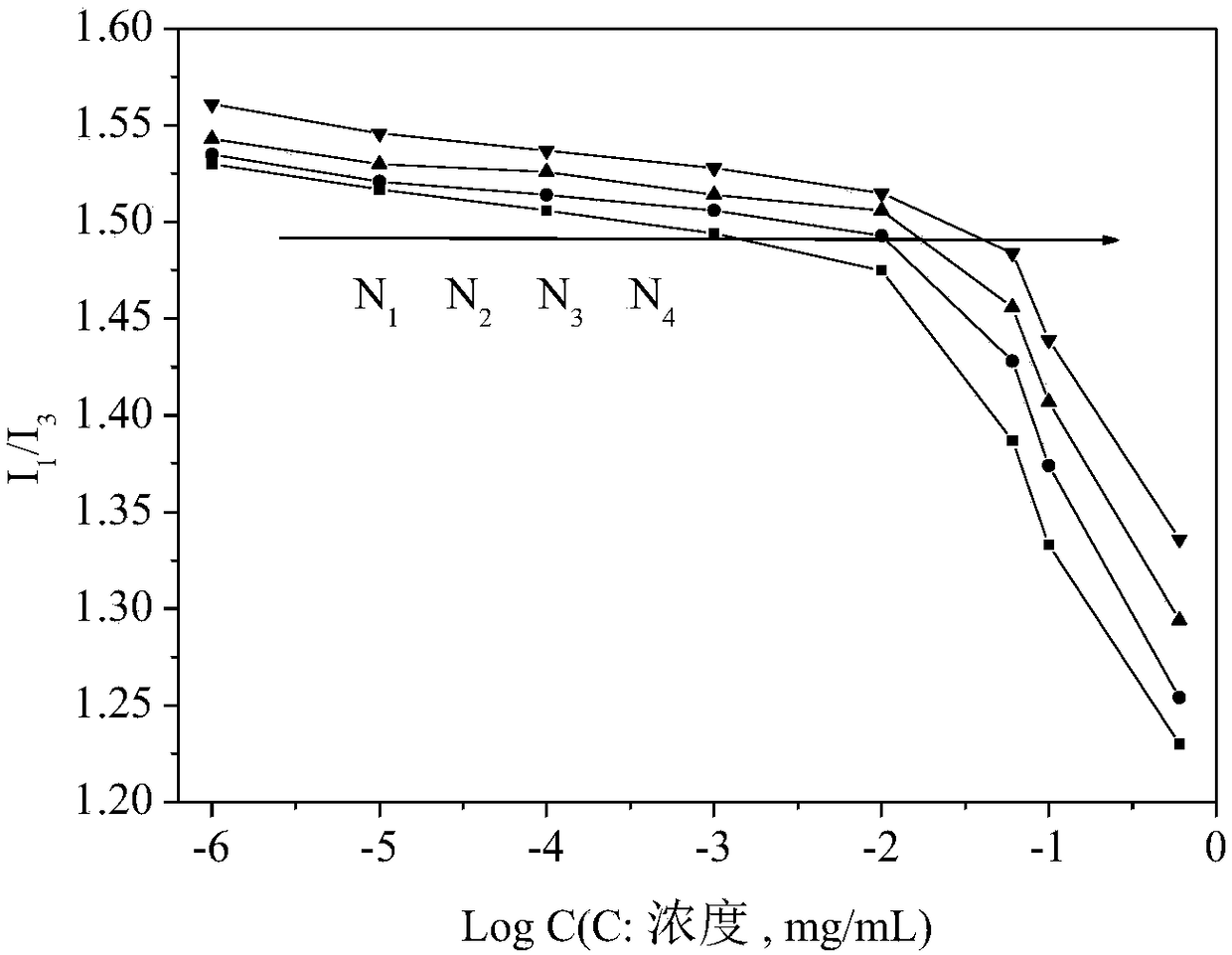
[0055] Measurement of charge-reversible reduction-sensitive reversibly cross-linked nanomicelles (CMC):
[0056] Dilute the charge-reversible reduction-sensitive reversible cross-linked nanomicelle solution obtained in Example 1 into a series of different concentrations, take 4 mL of the micellar solution of each concentration, and add 30 μL of it to a concentration of 1.622×10 -5 g / mL pyrene solution in acetone, protected from light, placed in a constant temperature shaking box under a nitrogen atmosphere, and incubated at 30°C for 24 hours. Measure the emission spectrum with a fluorescence spectrophotometer, set the fluorescence excitation wavelength λ=333nm, the scanning range λ=350-500nm, the excitation and emission slit widths are both 5nm, and the sample cell thickness is 1cm. By measuring a series of nanomicelle solutions with different concentrations at 373nm (I 1 ) and 384nm (I 3 ) fluorescence intensity, with the concentration logarithm as the X-axis, I 1 / I 3 Dr...
Embodiment 3
[0058] pH sensitivity of charge-reversible reduction-sensitive reversibly crosslinked nanomicelles:
[0059] The charge-reversible reduction-sensitive reversibly cross-linked nanomicelles obtained in Example 1 were adjusted to different pH values with 0.1 mol / L sodium hydroxide solution and hydrochloric acid solution, and the Zeta potential was measured with a Zeta potential analyzer. Results from Figure 4 It can be seen from the figure that when no lysine is added to the composition of the carrier material (that is, N 1 ), at pH 7.4, the nanomicelles were almost uncharged; and as the pH decreased, the positive charges on the surface of the nanomicelles increased. After adding the lysine component, the carboxyl group can be deprotonated under alkaline conditions, making the nano-micelle negatively charged; adjusting the distribution ratio of the three components can adjust the isoelectric point of the carrier. Glyceryl ether: lipoyl ethylenediamine: lysine molar ratio is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com