Spiro indolone compounds, a preparing method, a medicine composition and uses of the compounds
A spiroindolinone and compound technology, applied in the field of spiroindolinone compounds, can solve the problems of low selectivity and low inhibitory activity of antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
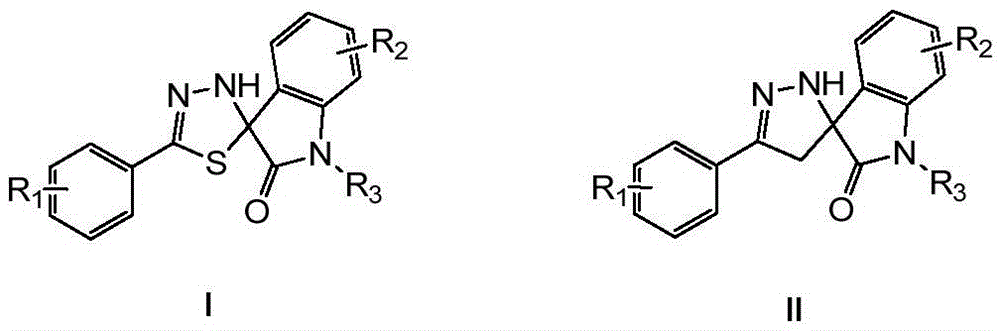
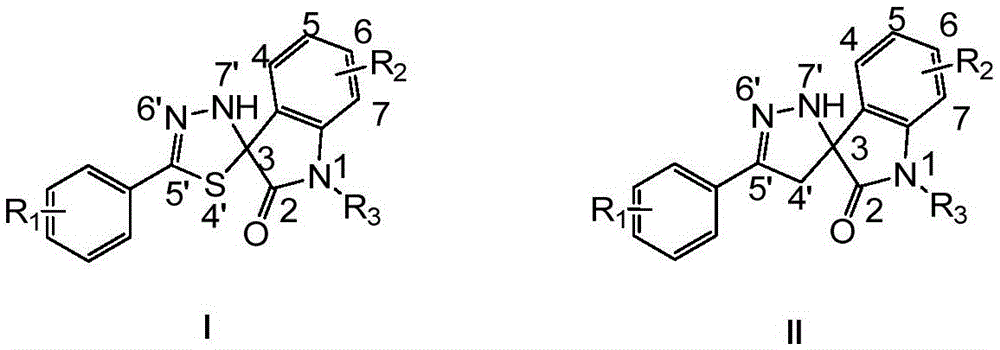
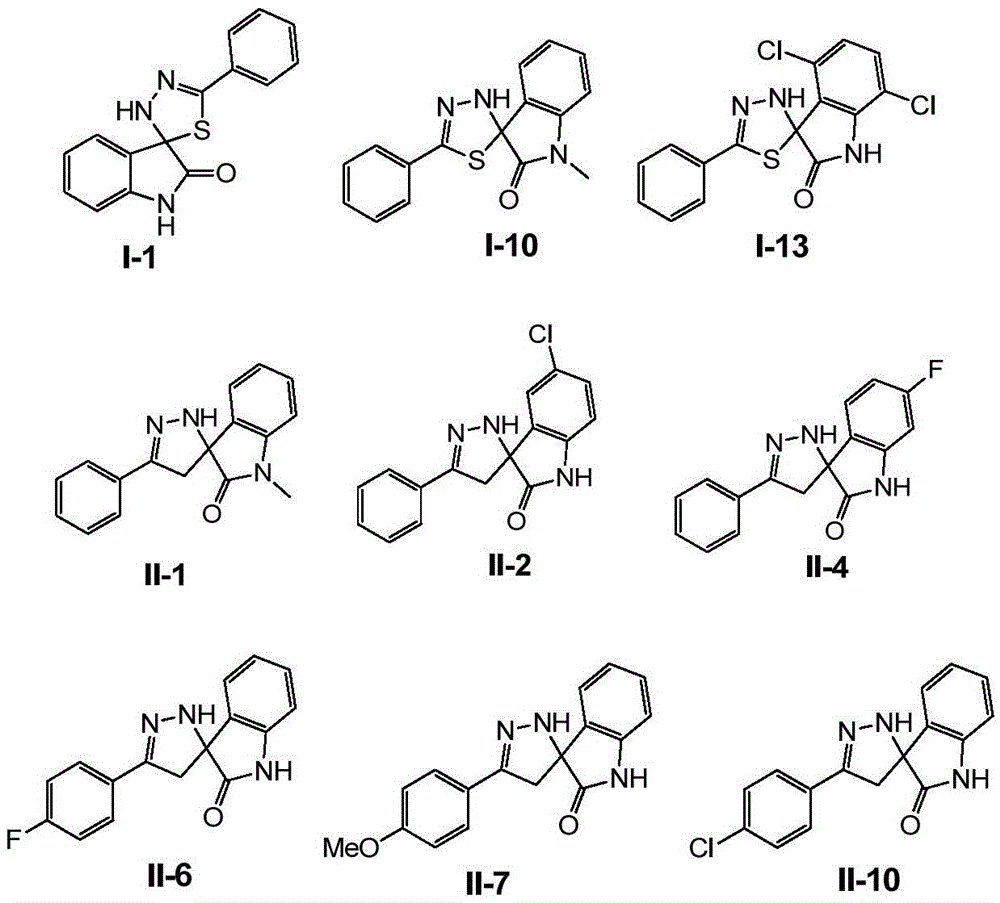
[0158] Example 1 Preparation of 5'-phenyl-3'H-spiro[indoline-3,2'-[1,3,4]thiadiazol]-2-one
[0159]
[0160] Thiobenzohydrazide (1.52g, 0.01mol) and isatin (1.47g, 0.01mol) were dissolved in ethanol (50ml), heated to 40°C for 1 hour. TLC monitored the completion of the reaction. The reaction solution was concentrated and filtered. The filtered solid was recrystallized from ethyl acetate to obtain the target product with a yield of 76% and a purity of 99% by HPLC.
[0161] 1 H NMR (300MHz, DMSO-d 6 )δ10.5(s,1H),8.88(s,1H),7.61-7.37(m,6H),7.31(t,J=7.2Hz,1H),7.05(t,J=7.8Hz,1H), 6.86(d,J=7.8Hz,1H); MS(ESI + )m / z 304(M+23)
Embodiment 2
[0162] Example 2 Preparation of 4-chloro-5'-phenyl-3'H-spiro[indoline-3,2'-[1,3,4]thiadiazol]-2-one
[0163]
[0164] Replace isatin with 4-chloroisatin, and the remaining required raw materials, reagents and preparation methods are the same as in Example 1 to obtain the product 4-chloro-5'-phenyl-3'H-spiro[indoline-3 ,2'-[1,3,4]thiadiazol]-2-one, yield 75%, HPLC purity 99%.
[0165] 1 H NMR (300MHz, DMSO-d 6 )δ10.84(s,1H),8.71(s,1H),7.56-7.40(m,5H),7.32(t,J=7.5Hz,1H),7.06(d,J=8.4Hz,1H), 6.86(d,J=8.4Hz,1H); MS(ESI + )m / z 338(M+23),354(M+39),370(M+55).
Embodiment 3
[0166] Example 3 Preparation of 5-chloro-5'-phenyl-3'H-spiro[indoline-3,2'-[1,3,4]thiadiazol]-2-one
[0167]
[0168] Replace isatin with 5-chloroisatin, and the rest of the required raw materials, reagents and preparation methods are the same as in Example 1 to obtain the product 5-chloro-5'-phenyl-3'H-spiro[indoline-3 ,2'-[1,3,4]thiadiazol]-2-one, yield 80%, HPLC purity 99%.
[0169] 1 H NMR (300MHz, DMSO-d 6 )δ10.66 (s, 1H), 8.90 (s, 1H), 7.60-7.40 (m, 6H), 7.36 (d, J = 8.4Hz, 1H), 6.88 (d, J = 8.4Hz, 1H); MS (ESI + )m / z 338(M+23).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com