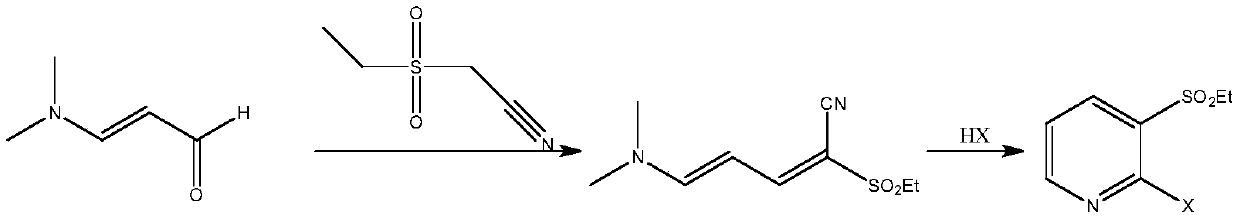
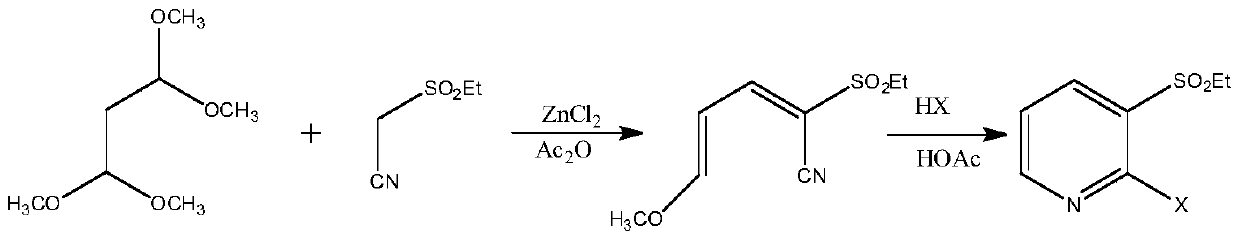
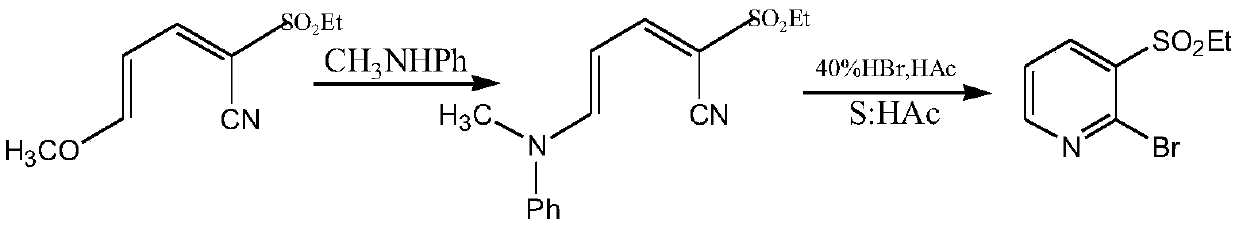
A method for synthesizing 2-halo-3-substituted hydrocarbylsulfonylpyridines and intermediates thereof by ionic liquid method
A technology for hydrocarbyl sulfonyl pyridine and hydrocarbyl, which is applied in the field of ionic liquid synthesis of 2-halogenated-3-substituted hydrocarbyl sulfonyl pyridine and its intermediates, and can solve the problems of difficult large-scale production, difficult treatment, and many three wastes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Preparation of 2-methylsulfonyl-5-(N,N-dimethyl)amino-2,4-pentadienenitrile
[0079] Add 59.6g (0.5mol) of methylcyanoethylsulfone, 50mL of N-butyl-N-ethylpiperidinium bromide, and 62mL (0.5mol) of 3-dimethylaminoacrolein into the reactor, mix well, and heat in microwave Reaction at a temperature of 55°C and incubated for 1 hour, TLC detection (petroleum ether:dichloromethane 1:2 development, sublimation iodine color development) 3-dimethylaminoacrolein has completely reacted, cooled to room temperature, organic solvent dichloromethane extraction 60mL ×3, the remaining phase ionic liquid was washed with water, dried in vacuum and reused. The organic phase was distilled off and the solvent dichloromethane was recovered to obtain 95.7 g of a light yellow solid with a melting point of 169-171° C. and a product yield of 95.6%. After HR-MS, 1 H NMR, 13 According to CNMR spectrum, the product is 2-methylsulfonyl-5-(N,N-dimethyl)amino-2,4-pentadienenitrile.
[00...
Embodiment 2
[0084] Example 2: Preparation of 2-ethylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile
[0085] Add 66.6g (0.5mol) of ethyl cyanoethyl sulfone, 50mL of N-ethyl-N-ethylpiperidinium chloride salt, and 124mL (1.0mol) of 3-diethylaminoacrolein into the reactor, mix well, and heat in a water bath Reaction at a temperature of 65°C and incubated for 10 hours, TLC detection (petroleum ether:dichloromethane 1:3 development, sublimation iodine color) ethyl cyanoethyl sulfone reaction is complete, cooled to room temperature, organic solvent toluene extraction 60mL×3, The remaining phase ionic liquid was washed with water, vacuum dried and reused. The organic phase was distilled off under reduced pressure to recover the solvent toluene to obtain 113.3 g of a light yellow solid with a melting point of 155-157° C. and a yield of 93.5%. The product was characterized by HR-MS, that is, 2-ethylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile. HR-MS(ESI):m / z Calcd for C 11 h 18 N 2 ...
Embodiment 3
[0086] Example 3: Preparation of 2-isopropylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile
[0087] Add 61mL (0.5mol) of isopropylcyanoethylsulfone, 50mL of N-ethyl-N-ethylpiperidinium chloride salt, and 62mL (0.5mol) of 3-diethylaminoacrolein into the reactor, mix well, and heat Reaction at a temperature of 63°C and incubated for 6 hours, TLC detection (petroleum ether:dichloromethane 1:2 development, sublimation iodine color development) 3-diethylaminoacrolein has completely reacted, cooled to room temperature, organic solvent toluene 60mL extraction 3 times , the remaining phase ionic liquid was washed with water, dried in vacuum and reused. The organic phase was evaporated under reduced pressure to remove the solvent toluene and recovered to obtain 118.2 g of light brown oil with a yield of 92.2%. The product was characterized by HR-MS, that is, 2-isopropylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile. HR-MS(ESI):m / z Calcd for C 12 h 20 N 2 o 2 S 279.3542[M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com