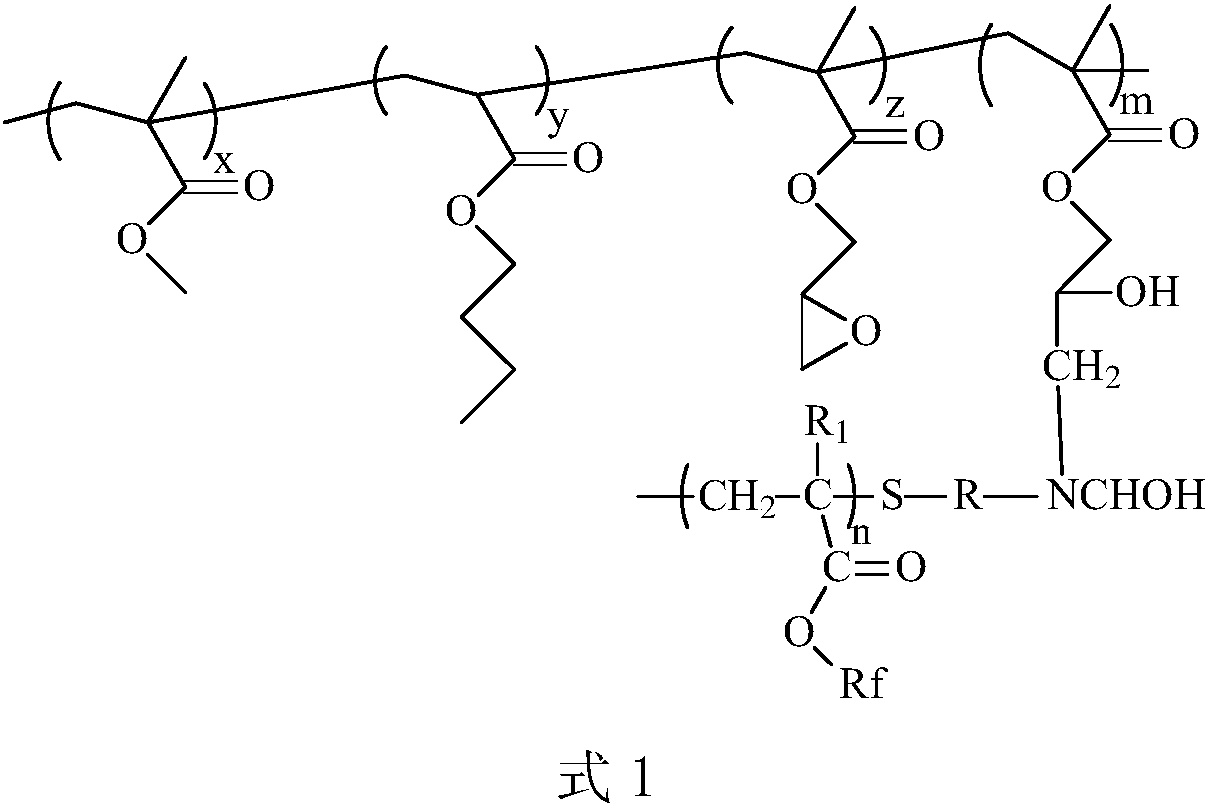
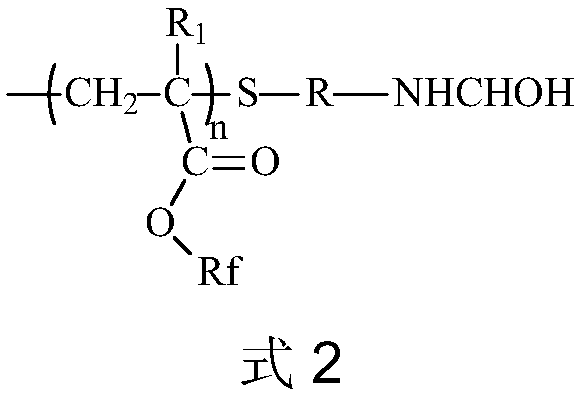
A kind of amino-terminated fluorine-containing prepolymer modified acrylate coating and preparation method thereof
A technology of acrylate and prepolymer, which is applied in the direction of coating, etc., can solve the problems of poor compatibility between fluorine-containing polymers and acrylate resins, the difficulty of compounding at the molecular level, and the increase in cost, and achieve the effect of low surface energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Preparation of amino-terminated fluorine-containing prepolymer: Add 20 parts by mass of hexafluorobutyl methacrylate and 40 parts by mass of N,N-dimethylformamide into a container, heat up to 60°C, and add 1 part by mass of Cysteamine hydrochloride, 0.6 parts by mass of azobisisobutyronitrile, stirred and reacted for 8 hours, added triethylamine to make the pH = 8, then added 0.8 parts by mass of 37% formaldehyde aqueous solution, and stirred thoroughly for 1 hour to obtain The amino-terminated fluorine-containing prepolymer solution is obtained by distilling off the solvent under reduced pressure to obtain an amino-terminated fluorine-containing prepolymer. FT-IR (Bruker TENSOR27 (Germany) Fourier infrared spectrometer) has N-H single-peak absorption peak and C-N absorption peak at 3379~3558cm-1 and 1200cm-1, respectively, and 1H-NMR (deuterated chloroform as solvent) at 2.8ppm The proton absorption peak of N-H appears at , indicating that the end of the product is...
Embodiment 2
[0047] (1) Preparation of amino-terminated fluorine-containing prepolymer: Add 40 parts by mass of hexafluorobutyl methacrylate and 80 parts by mass of N,N-dimethylformamide into a container, heat up to 60°C, add 4 parts by mass of Mercaptoethylamine, 0.8 parts by mass of azobisisobutyronitrile, stirred and reacted for 9 hours, added triethylamine to make the pH = 8, and then added 2 parts by mass of 38% formaldehyde aqueous solution, and stirred thoroughly for 0.5 hours to obtain the terminal amino group A fluorine-containing prepolymer solution, and the solvent is distilled off under reduced pressure to obtain an amino-terminated fluorine-containing prepolymer. FT-IR (Bruker TENSOR27 (Germany) Fourier infrared spectrometer) has N-H single-peak absorption peak and C-N absorption peak at 3364~3458cm-1 and 1190cm-1 respectively, 1H-NMR (deuterated chloroform as solvent) at 2.8ppm The proton absorption peak of N-H appears at , indicating that the end of the product is an amino g...
Embodiment 3
[0051] (1) Preparation of amino-terminated fluorine-containing prepolymer: Add 30 parts by mass of hexafluorobutyl methacrylate and 60 parts by mass of N,N-dimethylformamide into a container, heat up to 110°C, and add 0.6 parts by mass of Mercaptoethylamine, 0.1 parts by mass of azobisisobutyronitrile, stirred and reacted for 9 hours, diisopropylethylamine was added to make the pH = 8, and 1.5 parts by mass of formaldehyde solution with a mass concentration of 40% was added, and after fully stirred for 2 hours, the obtained The amino-terminated fluorine-containing prepolymer solution is obtained by distilling off the solvent under reduced pressure to obtain an amino-terminated fluorine-containing prepolymer. FT-IR (Bruker TENSOR27 (Germany) Fourier infrared spectrometer) has N-H single-peak absorption peak and C-N absorption peak at 3364~3458cm-1 and 1190cm-1 respectively, 1H-NMR (deuterated chloroform as solvent) at 2.8ppm The proton absorption peak of N-H appears at , indica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com