Recombinant adenovirus and construction method thereof
A technology of recombinant adenovirus and adenovirus, applied in the direction of virus, virus/phage, double-stranded DNA virus, etc., can solve the problems of independent enhancement, virus reversion, attenuated swine fever vaccine and attenuated PRRS vaccine can not be immune at the same time. , to achieve strong resistance, solve the problem of not being able to immunize at the same time, and reduce the number and cost of vaccinations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A. Amplification of classical swine fever virus E2 gene and PRRS virus GP5 gene
[0027] (1) Design of PCR amplification primers
[0028] Add Kpn I and Bgl II restriction endonuclease sites respectively in the upstream and downstream primers of the classical swine fever virus E2 gene, and the upstream and downstream primers of the classical classical swine fever virus E2 gene are designed as follows:
[0029]Upstream primer P1: 5'-AAAGGTACCATGAGGGGACAGATCGTGC-3'
[0030] Downstream primer P2: 5'-CCCAGATCTACCAGCGGCGAGTTGTTCTG-3'
[0031] In the upstream and downstream primers of the PRRS virus GP5 gene, respectively add Bgl II and Not I and a restriction endonuclease cutting site, the upstream and downstream primers of the PRRS virus GP5 gene are designed as follows:
[0032] Upstream primer P1: 5'-AAAAGATCTATGTTGGGCAAGTGCTTGAC-3'
[0033] Downstream primer P2: 5'-CCCGCGGCCGCCTAGAGACGAGCCCATTG-3'
[0034] (2) In vitro amplification of classical swine fever virus E2 g...
Embodiment 2
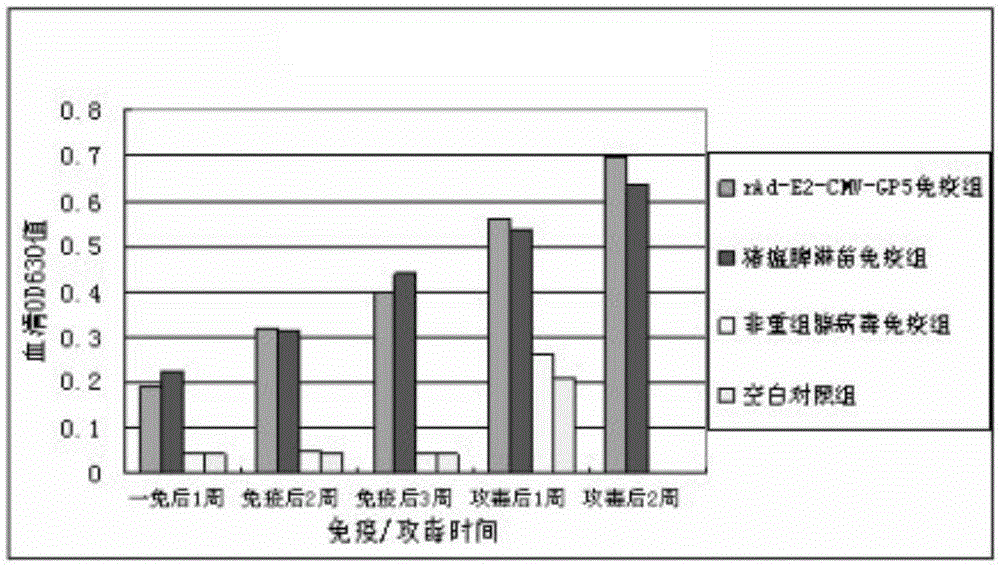
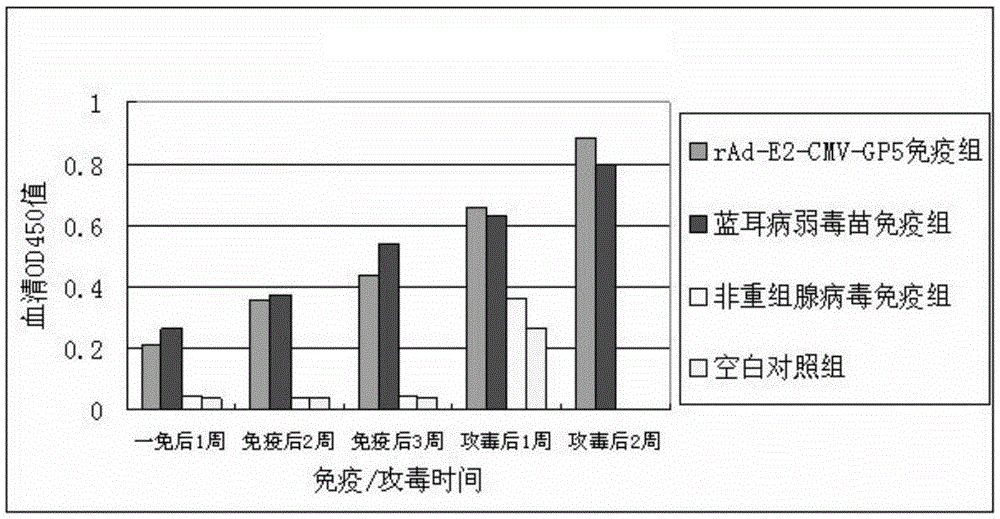
[0124] 1. rAd-E2-CMV-GP5 immune protection test
[0125] (1) Grouping of experimental animals
[0126] 24 pigs were randomly divided into 4 groups, the first group was rAd-E2-CMV-GP5 immunized group, 8 pigs. The second group is the immunization group of swine fever spleen drenching vaccine and PRRS attenuated vaccine, with 8 animals. The third group is the non-recombinant adenovirus immunization group, with 4 heads. The fourth group is the blank control group with 4 heads.
[0127] (2) Immunity and attack
[0128] The immunization doses of the recombinant adenovirus group and the non-recombinant adenovirus group were both 1.2×109pfu (about 2mL), which were injected into the neck muscle and abdomen subcutaneously at multiple points; The dosage was carried out according to the instructions of the vaccine; the blank control was immunized with the DMEM medium cultured with the recombinant adenovirus, 2 mL / head. All test pigs were challenged by intramuscular injection, eye dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com