A transport bed methanation catalyst, a preparing method thereof and applications of the catalyst
A catalyst, bed methane technology, used in physical/chemical process catalysts, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. The effect of improved strength and wear resistance, good flowability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
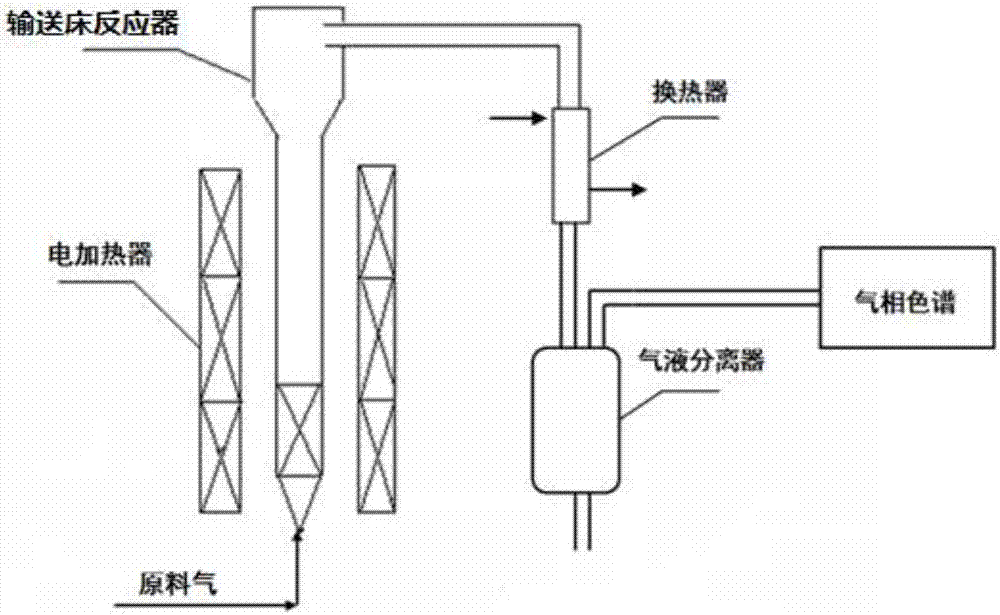
Image
Examples
Embodiment 1
[0028] The specific preparation process of the catalyst of this embodiment is as follows:
[0029] 1) 88.80 grams of Mg(NO 3 ) 2 ·6H 2 O, 14.07 g Zr(NO 3 ) 4 ·5H 2 O, 80.96 g Ni(NO 3 ) 2 ·6H 2 O (AR grade) mixed with 100ml deionized water to make a solution;
[0030] 2) 50 g of α-Al with a particle size distribution of 20-180 μm after roasting treatment 2 o 3 The microspheres were immersed in the above solution and placed in a water bath at 40°C for 2 hours;
[0031] 3) The solution prepared in step 2) was separated by filtration, and the obtained particles were dried at 80° C. for 12 hours.
[0032] 4) The particles obtained in step (3) were calcined at 600° C. for 6 hours to obtain the catalyst of this example.
[0033] Among them, α-Al 2 o 3 The microspheres were calcined at 900°C for 6h in advance.
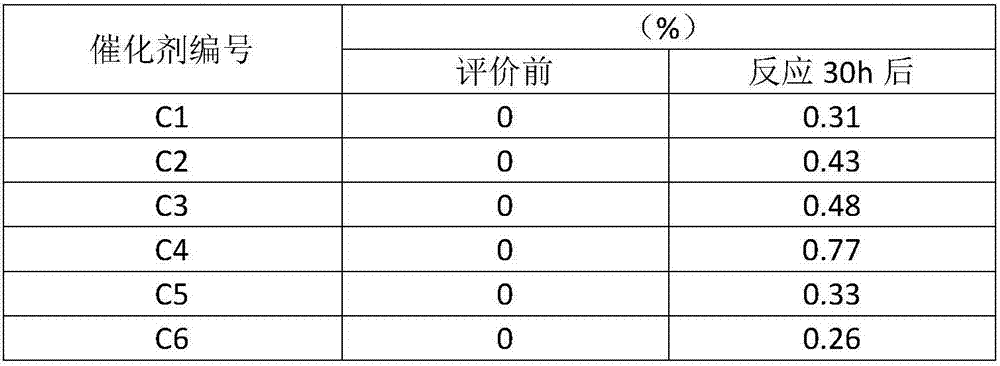
[0034] The catalyst prepared in this embodiment consists of: 15 parts of NiO, 1 part of ZrO 2 , 5 parts of MgO and 79 parts of Al 2 o 3 , recorded as C1.
Embodiment 2
[0036] The specific preparation process of the catalyst of this embodiment is as follows:
[0037] 1) 85.50 grams of Mg(NO 3 ) 2 ·6H 2 O, 33.78 g Ce(NO 3 ) 3 ·6H 2O (AR grade), 77.88 grams of Ni (NO 3 ) 2 ·6H 2 O mixed and added to 100ml deionized water to make a solution.
[0038] 2) 50 g of α-Al with a particle size distribution of 30-170 μm after roasting treatment 2 o 3 The microspheres were immersed in the above solution and placed in a water bath at 60°C for 2.5 hours;
[0039] 3) The solution prepared in step 2) was separated by filtration, and the obtained particles were dried at 100° C. for 8 hours.
[0040] 4) The particles obtained in step 3) were calcined at 700° C. for 6 hours to obtain the catalyst of this example.
[0041] Among them, α-Al 2 o 3 The microspheres were pre-calcined at 1000 °C for 5 h.
[0042] The catalyst prepared in this embodiment consists of: 15 parts of NiO, 5 parts of CeO 2 , 5 parts of MgO and 75 parts of Al 2 o 3 , record...
Embodiment 3
[0044] The specific preparation process of the catalyst of this embodiment is as follows:
[0045] 1) 88.84 grams of Mg(NO 3 ) 2 ·6H 2 O, 36.88 g La(NO 3 ) 3 (AR grade), 96g Ni(NO 3 ) 2 ·6H 2 O mixed and added to 100ml deionized water to form a solution;
[0046] 2) 50 g of α-Al with a particle size distribution of 50-160 μm after roasting treatment 2 o 3 The microspheres were immersed in the above solution and placed in a water bath at 70°C for 3.5 hours;
[0047] 3) The solution prepared in step 2) was separated by filtration, and the obtained particles were dried at 110° C. for 10 h;
[0048] 4) The particles obtained in step 3) were calcined at 600° C. for 6 hours to obtain the catalyst of this example.
[0049] Among them, α-Al 2 o 3 The microspheres were pre-calcined at 1100 °C for 4 h.
[0050] The catalyst prepared in this embodiment consists of: 18 parts of NiO, 5 parts of La 2 o 3 , 5 parts of MgO and 72 parts of Al 2 o 3 , recorded as C3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com