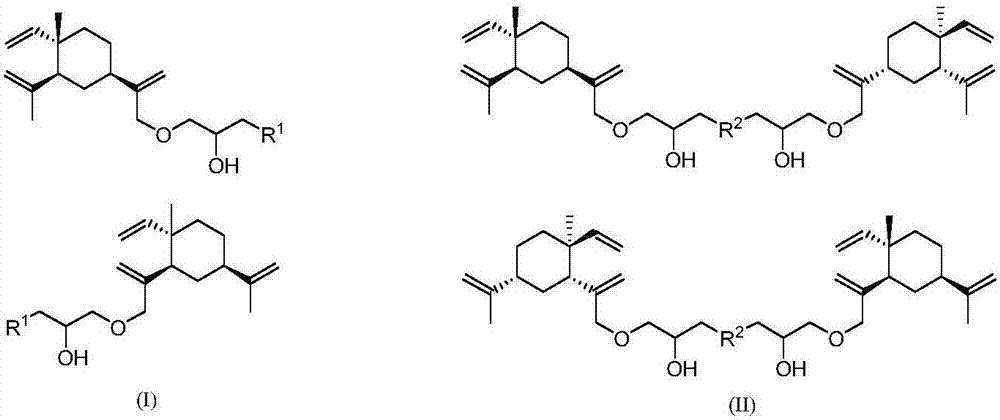
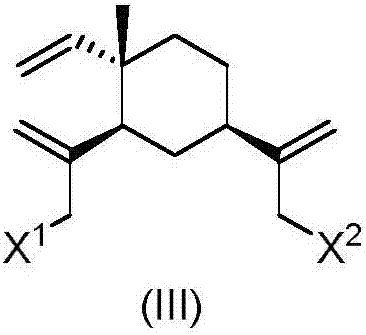
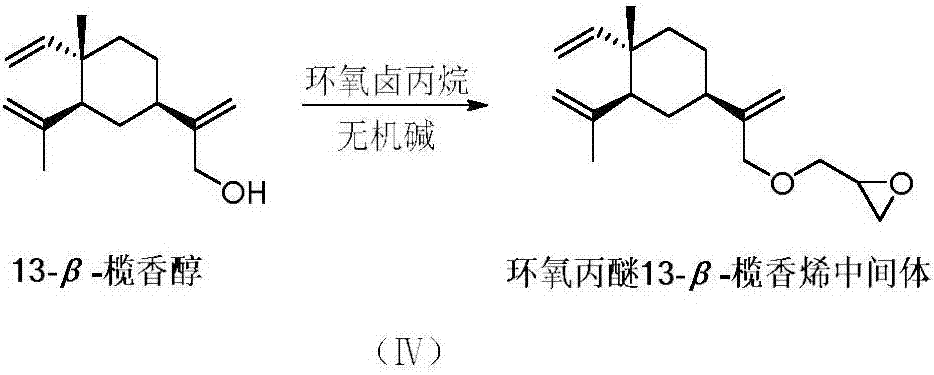
Isopropanolamine substituted beta-elemene derivative and preparation method and application thereof
A technology of isopropanolamine and elemene, which is applied in the field of preparation of antitumor drugs, can solve the problems of low bioavailability and limited wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Synthesis of N,N-diethylisopropanolamine-β-elemene
[0102] Dissolve 0.5mmol glycidyl ether β-elemene and 1.5mmol diethylamine in 1mL methanol, add 0.05mmol Zn(ClO 4 ) 2 ·6H 2 O, stirred at 80 °C for 1 h. Cool to room temperature, add water, extract with dichloromethane, dry over anhydrous sodium sulfate, filter, evaporate dichloromethane under reduced pressure, and use dichloromethane:methanol=40:1 column chromatography to obtain a light yellow liquid product with a yield of 66%. 1 H NMR (300MHz, CDCl 3 )δ5.75(dd, J=17.8, 10.5Hz, 1H), 4.94(s, 1H), 4.91(s, 1H), 4.85(d, J=3.8Hz, 1H), 4.81(s, 1H), 4.75(s, 1H), 4.51(s, 1H), 4.25–4.12(m, 2H), 4.02–3.86(m, 2H), 3.58–3.41(m, 2H), 3.38–3.21(m, 4H), 3.20–3.14(m, 2H), 2.02–1.84(m, 2H), 1.64(s, 3H), 1.63–1.49(m, 3H), 1.48–1.28(m, 9H), 0.93(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ 149.55, 149.30, 147.06, 111.67, 110.06, 109.52, 73.29, 70.93, 64.45, 55.21, 52.13, 48.32, 40.92, 39.34, 39.27, 32.68, 26.59, 24.35, 16.06, 8.3
Embodiment 2
[0104] Synthesis of tetrahydropyrrole-β-elemene with isopropanol
[0105] Tetrahydropyrrole was used instead of diethylamine, and other conditions were the same as in Example 1 to obtain a light yellow liquid product with a yield of 73%. 1H NMR (300MHz, CDCl3) δ5.82(dd, J=17.8, 10.5Hz, 1H), 5.02(s, 1H), 4.98(s, 1H), 4.92(d, J=3.9Hz, 1H), 4.88 (s, 1H), 4.82(s, 1H), 4.58(s, 1H), 4.38(s, 1H), 4.25–4.12(m, 1H), 4.07–3.93(m, 2H), 3.75–3.12(m , 8H), 2.27–2.09(m, 4H), 2.06–1.87(m, 2H), 1.71(s, 3H), 1.68–1.50(m, 3H), 1.52–1.30(m, 3H), 1.00(s , 3H); 13C NMR (75MHz, CDCl3) δ149.56, 149.34, 147.09, 111.67, 109.97, 109.53, 73.22, 70.94, 65.52, 58.18, 54.98, 52.11, 40.88, 39.34, 39.28, 32.66, 24.35 , 16.06.
Embodiment 3
[0107] Synthesis of isopropanol piperidine-β-elemene
[0108] Piperidine was used instead of diethylamine, and other conditions were the same as in Example 1 to obtain a light yellow liquid product with a yield of 68%. 1 H NMR (300MHz, CDCl 3 )δ5.82(dd, J=17.8, 10.5Hz, 1H), 5.02(s, 1H), 4.98(s, 1H), 4.92(d, J=4.0Hz, 1H), 4.88(s, 1H), 4.82(s, 1H), 4.58(s, 1H), 4.38(s, 1H), 4.30–4.20(m, 1H), 4.08–3.94(m, 2H), 3.57–3.44(m, 2H), 3.33– 3.08(m, 6H), 2.08–1.81(m, 6H), 1.71(s, 3H), 1.69–1.54(m, 5H), 1.53–1.41(m, 3H), 1.00(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ149.58, 149.41, 147.08, 111.66, 110.01, 109.50, 73.24, 71.25, 64.48, 60.21, 54.25, 52.14, 40.86, 39.35, 39.29, 32.65, 26.56, 24.35, 22.909, 21.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com