A fusion polypeptide with anti-cerebral ischemia effect and its application
An ischemic and cerebrovascular disease technology, applied to a polypeptide with anti-cerebral ischemia effect and its application field, can solve problems such as inaccurate curative effect and adverse reactions, and achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1. Preparation of polypeptide (YGRKKRRQRRRGDPV)
[0023] 1. Peptide synthesis process: Peptide synthesis process: first soak the resin (Fmoc-Val-Wang Resin) in DCM for 5-10 minutes, wash it with DMF for 5 times, deprotect it with 20% hexahydropyridine for 10-20 minutes, and use DMF Wash 5 times, then add protected amino acid raw materials, condensing agent, etc. to condense for 30 minutes, wash 5 times with DMF, then deprotect—wash—condense—clean, finally deprotect, wash, drain, cut with TFA, and use cutting fluid Precipitate with ether, wash with ether 2-3 times, and drain. This is a general process of the whole peptide synthesis.
[0024] 2. Polypeptide synthesis starts from the C-terminal, that is, synthesis from right to left. First, this sequence YGRKKRRQRRRGDPV is synthesized with conventional amino acid raw materials. The amino acid raw materials used are Fmoc-Pro-OH, Fmoc-Asp(otbu)- OH, Fmoc-Gly-OH, Fmoc-Gln(Trt)-OH, Fmoc-Arg(pbf)-OH, Fmoc-Lys(Boc)-OH, ...
Embodiment 2
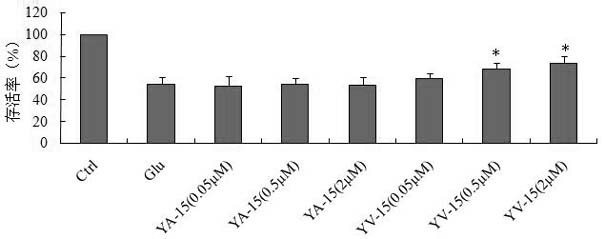
[0025] Example 2. Effect of polypeptide on glutamic acid excitotoxicity
[0026] Fetal mouse cortical neuron cells cultured in vitro for 11 days were washed with HBSS for 3 times, and incubated with 50 μM glutamic acid for 20 min. Distilled water, YV-15 or YA-15 were given respectively during the incubation with glutamic acid, and the peptides used The concentrations were 0.05, 0.5 and 2 μmol / L. After the incubation, all the drugs were removed, the plate was washed 3 times with HBSS, the maintenance medium was added, and placed in 37°C containing 5% CO 2 The culture was continued for 24 h in an incubator, and the damage of the cells was detected by the MTT assay.
Embodiment 3
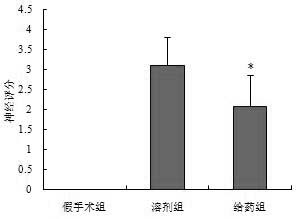
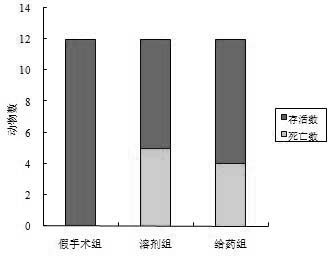
[0027] Example 3. Effects of polypeptides on neurobehavioral scores and mortality of animals after cerebral ischemia
[0028] 1. Establishment and administration of rat MCAO model: Sprague Dawley (SD) rats, male, body weight (300±20) g. The cerebral ischemia model of middle cerebral artery occlusion (MCAO) was established by the internal carotid artery suture method. Rats were anesthetized with 10% chloral hydrate (300 mg·kg - 1 , i.p. ), fixed on the operating table in the supine position, and a median incision was made in the neck to separate the right common carotid artery, external carotid artery, and internal carotid artery. , Gently strip the vagus nerve and ligate all branches on the external and internal carotid arteries. External carotid artery incision, insert a nylon fishing line with an outer diameter of 0.28 mm (the head end is treated with silicone resin to make it smooth), turn around the bifurcation of the common carotid artery and enter the internal carotid a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com