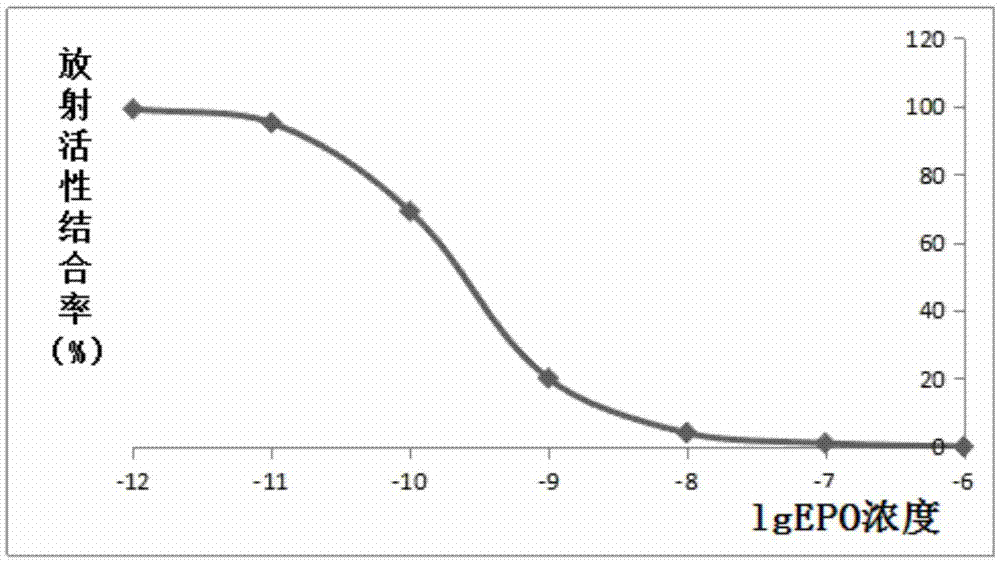
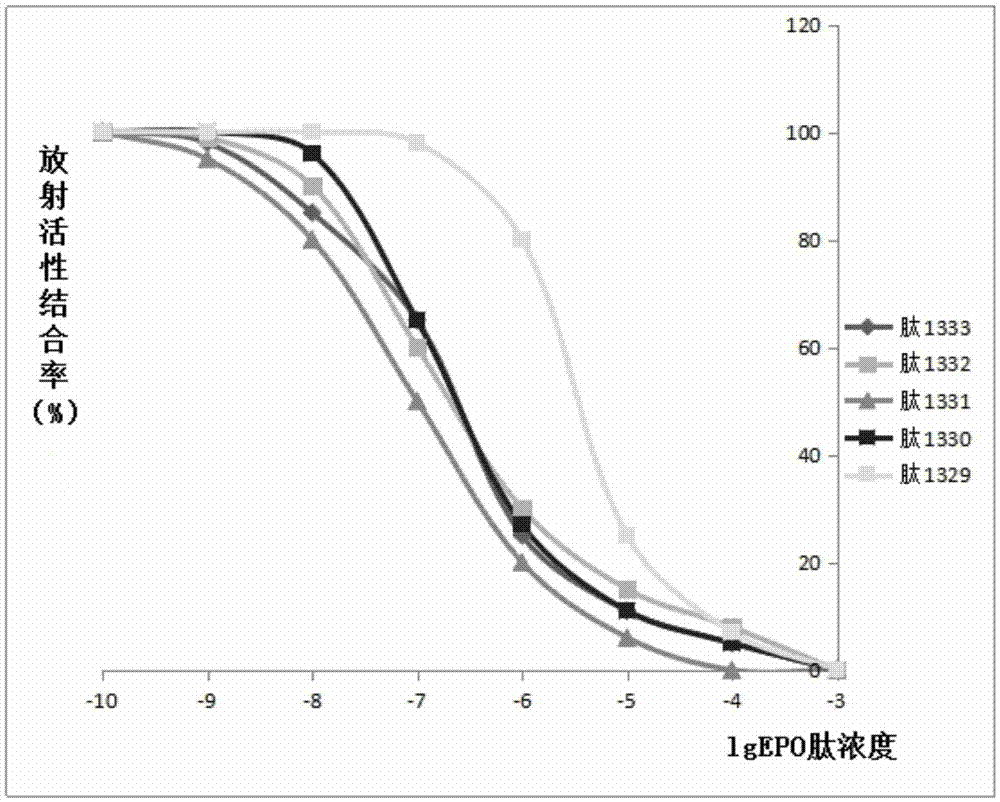
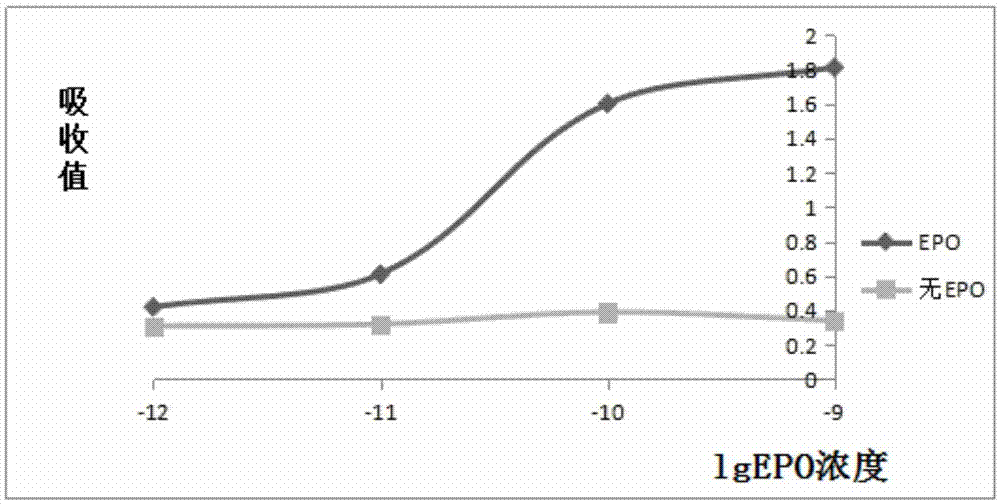
Hemopoietin peptide, hemopoietin peptide derivative, hemopoietin peptide polymer, preparation method of hemopoietin peptide and application of hemopoietin peptide
A technology of erythropoietin and peptide derivatives, which is applied in the field of protein peptides, can solve the problems of short half-life, low biological activity, and poor clinical compliance, and achieve the effect of low side effects and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The present invention also provides a preparation method of erythropoietin peptide, but not limited to the following methods:
[0057] The route of the present invention is described by taking the following sequence as an example: H-Leu-Tyr-Ala-Cys-Lys-Met-Gly-Pro-Ile-Thr-Trp-Val-Cys-Pro-Leu-Arg-OH
[0058] Using solid-phase synthesis method, using CTC resin / King resin as the starting resin, sequentially coupling amino acids with N-terminal Fmoc protection and side chain protection according to the peptide sequence of the erythropoietin peptide main chain; finally, the peptide resin was cleaved, The target compound was obtained after purification and lyophilization.
[0059] For this reason the present invention provides the synthetic method of erythropoietin peptide, and its steps are as follows:
[0060] Step 1, in the presence of an activator system, the Fmoc-Arg (Pbf)-resin is obtained by coupling the resin solid phase support and Fmoc-Arg (Pbf)-OH;
[0061] Step ...
Embodiment 1
[0137] Synthesis of erythropoietin peptide whose sequence is SEQ ID No.2: H-Leu-Tyr-Ala-Cys-Lys-Met-Gly-Pro-Ile-Thr-Trp-Val-Cys-Pro-Leu-Arg Synthesis of -OH
[0138] (1) Weigh 0.1 mmol of Fmoc-Arg(Pbf)-King resin with a degree of substitution of 0.52 mmol / g, add it to a solid-phase reaction column, wash it once with DMF, and swell the Fmoc-Arg(Pbf)-King resin with DMF After 30 minutes, use a mixed solution of DMF:pyridine with a volume ratio of 4:1 to remove Fmoc protection, then wash with DMF for 6 times, weigh 0.5mmol of Fmoc-Leu-OH, and add 0.5mmol of HOBt with a volume ratio of 1:1. The mixed solution of DCM and DMF was activated by adding 80μl DIC (0.5mmol) in an ice-water bath, and then added to the above-mentioned reaction column equipped with resin. If it is transparent, it means that the reaction is complete; if the resin is colored, it means that the reaction is incomplete and needs to be reacted for another 1 hour. This judgment standard is applicable to the ninhyd...
Embodiment 2
[0143] Synthesis of erythropoietin peptide whose sequence is SEQ ID No. 3: H-Leu-Tyr-Ala-Cys-Tyr-Met-Gly-Pro-Ile-Thr-Trp-Val-Cys-Pro-Leu-Arg Synthesis of -OH
[0144] (1) Weigh 0.1 mmol of Fmoc-Arg(Pbf)-King resin with a degree of substitution of 0.52 mmol / g, add it to a solid-phase reaction column, wash it once with DMF, and swell the Fmoc-Arg(Pbf)-King resin with DMF After 30 minutes, use a mixed solution of DMF:pyridine with a volume ratio of 4:1 to remove Fmoc protection, then wash with DMF for 6 times, weigh 0.5mmol of Fmoc-Leu-OH, and add 0.5mmol of HOBt with a volume ratio of 1:1. The mixed solution of DCM and DMF was activated by adding 80μl DIC (0.5mmol) in an ice-water bath, and then added to the above-mentioned reaction column equipped with resin. If it is transparent, it means that the reaction is complete; if the resin is colored, it means that the reaction is incomplete and needs to be reacted for another 1 hour. This judgment standard is applicable to the ninhy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com