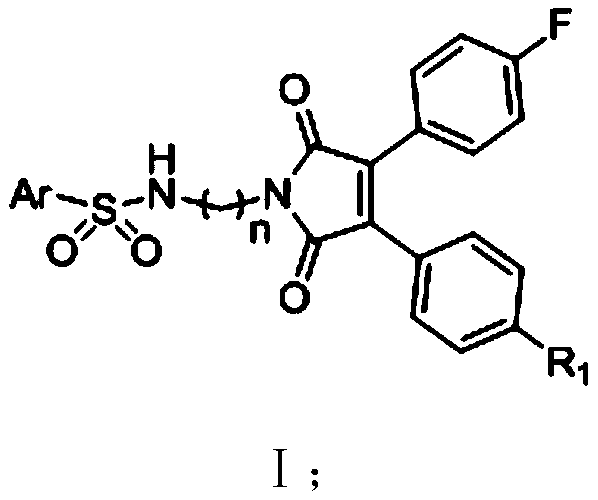
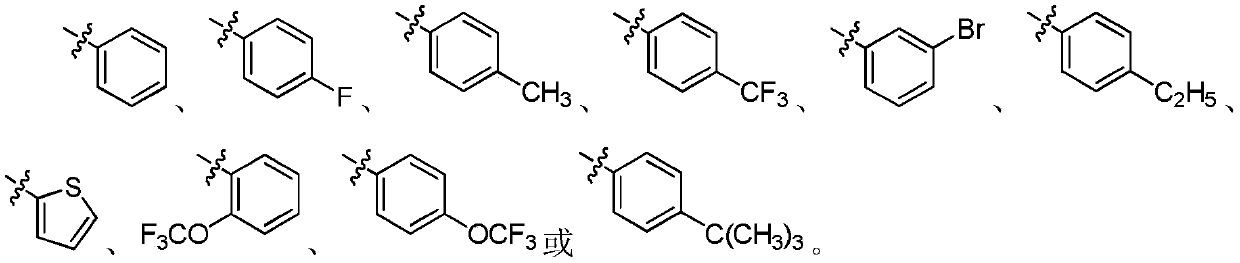
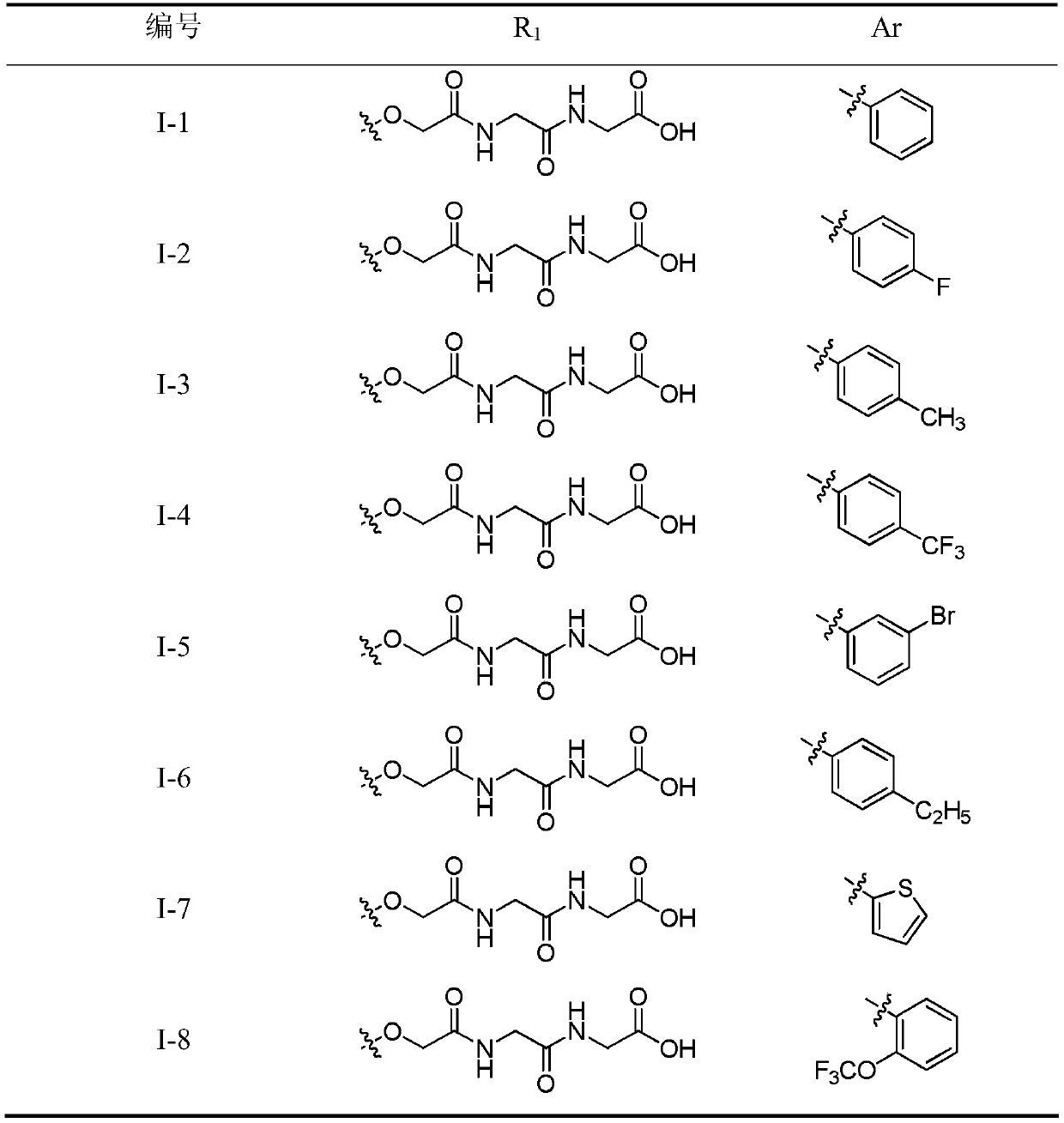
A kind of 3,4-diarylmaleimide derivative and its preparation method and application
A technology of maleimide and derivatives, which is applied in the field of 3,4-diarylmaleimide derivatives and its preparation, and can solve problems such as safety risks and poor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1: Preparation of 2-bromo-1-(4-fluorophenyl)ethanone
[0087]Dissolve 20g (144.7mmol) of p-fluoroacetophenone in 200mL of acetonitrile, slowly add 23.12g (144.7mmol) of Br 2 50 mL of acetonitrile solution, stirred overnight at room temperature. After the reaction was complete, the reaction solution was concentrated and removed, redissolved in ethyl acetate, washed three times with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure to remove the solvent. 1 mL of ethyl acetate was slurried and filtered to obtain 28 g of white solid, yield: 89.2%, mp: 40-41°C.
[0088] 1 H-NMR (300M, CDCl 3 ):4.40(s,2H,-CH 2 -),7.17(m,2H,Ar-H),8.02(m,2H,Ar-H); MS:calcd.m / z[M]215.96,found m / z[M-H] - 215.0.
Embodiment 2
[0089] Example 2: Preparation of 2-azido-1-(4-fluorophenyl)ethanone
[0090] Dissolve 31.59g (146mmol) of 2-bromo-1-(4-fluorophenyl)ethanone in 150mL of methanol, and slowly add 50mL of 11.38g (175mmol) of NaN at 0°C 3 Aqueous solution, stirred overnight at room temperature. After the reaction was complete, 50 mL of water was slowly added dropwise at 0°C, a large amount of white solid precipitated, filtered and dried to obtain 25.25 g, yield: 96.6%.
[0091] 1 H-NMR (300M, CDCl 3 ):4.52(s,2H,-CH2-),7.17(m,2H,Ar-H),7.94(m,2H,Ar-H); MS:calcd.m / z[M]179.05,found m / z[M+H] + 180.0.
Embodiment 3
[0092] Embodiment 3: Preparation of 2-amino-1-(4-fluorophenyl)ethanone hydrochloride
[0093] Dissolve 25.25g (141mmol) of 2-azido-1-(4-fluorophenyl)ethanone in 100mL of methanol, slowly add 42.85g (423mmol) of concentrated hydrochloric acid (concentration 36%) dropwise, add 2g of Pd / C , catalytic hydrogenation at room temperature. After the reaction is complete, filter the Pd / C, distill off the solvent under reduced pressure, slurry with 50mL ether, and filter to obtain 22.72g of white solid, yield: 85%, mp: 213-215°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com