Diarrhea pathogenic bacteria multiple gene detection system and its kit and application
A technology for genetic detection and pathogenic bacteria, which is applied in the fields of biochemical equipment and methods, microbial determination/inspection, resistance to vector-borne diseases, etc., can solve the problem that the detection and diagnosis methods of diarrhea pathogenic bacteria cannot meet clinical needs and cannot accurately identify various pathogenic bacteria. , the inability to diagnose the basis of pathogenic bacteria and other problems, to achieve the effect of low-cost pathogenic diagnosis, low cost and good convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. The composition of the kit
[0052] The diarrhea pathogen multiple gene detection kit of the present embodiment detection kit comprises: primer mixture, PCR buffer (5×PCR Buffer), MgCl 2 solution, dNTPs, hot-start DNA polymerase and reverse transcriptase mix, UDG enzyme, positive and negative controls.
[0053] PCR buffer, hot-start DNA polymerase and reverse transcriptase mix were all from Qiagen (Catalog No.: 210212).
[0054] The hot-start DNA polymerase and reverse transcriptase are mixed together to form a mixed solution, wherein the concentration of the hot-start DNA polymerase in the reaction system is 0.1U / μL, and the concentration of the reverse transcriptase in the mixed solution is 0.1U / μL.
[0055] The positive control solution is a plasmid mix that includes all gene targets of interest.
[0056] The negative control solution was nuclease-free ultrapure water.
[0057] The primer mixture includes: the nucleotide sequence of the forward primer and the n...
Embodiment 2
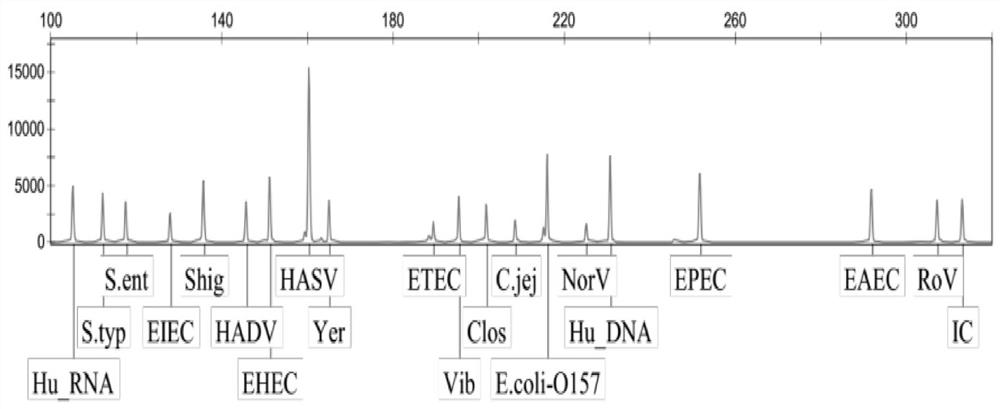
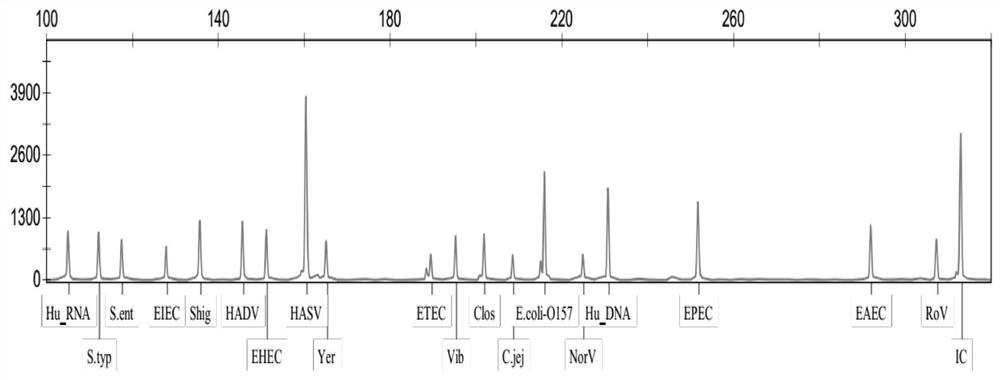
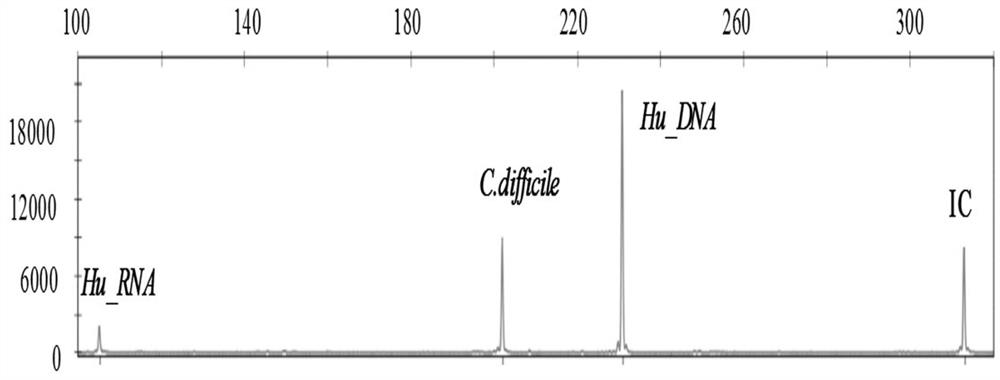
[0107] The rest of the diarrhea pathogenic bacteria multiple gene detection kit of the present embodiment is the same as that of Example 1, except that the primer mixture only includes: respectively for Campylobacter jejuni, Shigella, Clostridium difficile, Salmonella enteritidis , Salmonella typhimurium, Enterotoxigenic Escherichia coli, Enterohaemorrhagic Escherichia coli, Enteropathogenic Escherichia coli, Enteroadhesive Escherichia coli, Enteroinvasive Escherichia coli, Escherichia coli Forward and reverse PCR amplification primers for detection of bacteria O157, Vibrio, and Yersinia enterocolitica, and forward and reverse PCR amplification primers for detection of human DNA internal reference and system quality control internal reference. It is used for the simultaneous detection of 13 common pathogenic bacteria in infectious diarrhea.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com