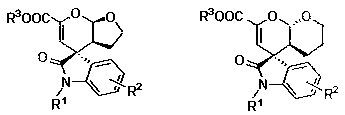
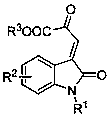
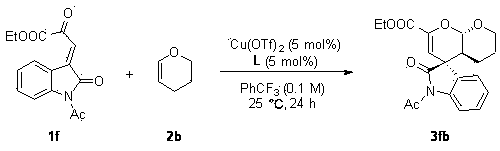
A chiral spiro-oxindole dihydropyran derivative and its synthesis method
A spirocyclic oxindole dihydropyran derivative and technology for a synthesis method are applied in the field of chiral spirocyclic oxindole dihydropyran derivatives and their catalytic synthesis, and achieve high yield, enantioselectivity and Excellent diastereoselectivity and high compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Under nitrogen atmosphere, Cu(OTf) 2 (0.9 mg, 0.0025 mmol, 2.5 mol%), L (1.84 mg, 0.0025 mmol, 2.5 mol%) were placed in anhydrous benzotrifluoride (1 mL), stirred at room temperature for 1 hour, and reactant 1a (25.9 mg, 0.1 mmol) and 2b (16.8 mg, 0.2 mmol, 2 equiv), reacted at room temperature for 24 hours until the substrate 1a disappeared, and the reaction system was directly separated by petroleum ether / ethyl acetate (3 / 1) column chromatography to obtain 32.2 mg White solid 3ab, white solid, 94% yield, 161–162 °C.
[0033] The product 3ab was analyzed and the results were as follows: >99:1 dr , 94% ee [Daicel Chiralcel AD-H, hexanes / i -PrOH = 80 / 20, flow rate: 1.0 mL·min –1 , λ = 254.4 nm, t (major)=12.087, t (minor)= 15.133]; [ α ]25 D = 148.9 (c 0.14, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 7.31 (td, J = 7.6, 1.7 Hz, 1H), 7.16 – 7.02 (m, 2H), 6.84 (d, J =7.8 Hz, 1H), 5.67 (s, 1H), 5.60 (d, J = 8.9 Hz, 1H), 4.23 (qq, J = 7.5, 3.7Hz, 2...
Embodiment 2
[0036]
[0037] Under nitrogen atmosphere, Cu(OTf) 2 (1.8 mg, 0.005 mmol, 5 mol%), L (3.69 mg, 0.005 mmol, 5 mol%) were placed in anhydrous benzotrifluoride (1 mL), stirred at room temperature for 1 hour, and reactant 1b (24.5 mg, 0.1 mmol) and 2b (33.6 mg, 0.4 mmol, 4 equiv), reacted at room temperature for 24 hours until the substrate 1b disappeared, and the reaction system was directly separated by petroleum ether / ethyl acetate (3 / 1) column chromatography to obtain 29.9 mg White solid 3bb, white solid, 91% yield, 140–141 °C.
[0038] Product 3bb is analyzed, and the results are as follows: 96:4 dr , 93% ee [Daicel Chiralcel AD-H, hexanes / i -PrOH = 80 / 20, flow rate: 1.0 mL·min –1 , λ = 254.4 nm, t (major)=13.307, t (minor)= 18.928]; [ α ]25 D = 191.7 (c 0.1, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 7.33 (td, J = 7.5, 1.8 Hz, 1H), 7.16 – 7.06 (m, 2H), 6.85 (d, J =7.8 Hz, 1H), 5.70 (s, 1H), 5.63 (d, J = 8.9 Hz, 1H), 4.09 (dd, J = 11.8, 4.6Hz, 1H), 3.80 (s,...
Embodiment 3
[0041]
[0042] Under nitrogen atmosphere, Cu(OTf) 2 (0.9 mg, 0.0025 mmol, 2.5 mol%), L (1.84 mg, 0.0025 mmol, 2.5 mol%) were placed in anhydrous benzotrifluoride (1 mL), stirred at room temperature for 1 hour, and reactant 1c (28.9 mg, 0.1 mmol) and 2b (16.8 mg, 0.2 mmol, 2 equiv), reacted at room temperature for 24 hours until the substrate 1c disappeared, and the reaction system was directly separated by petroleum ether / ethyl acetate (3 / 1) column chromatography to obtain 34.6 mg White solid 3cb, white solid, 93% yield, 63–64 °C.
[0043] The product 3cb was analyzed and the results were as follows: >99:1 dr , 93% ee [Daicel Chiralcel AD-H, hexanes / i -PrOH = 80 / 20, flow rate: 1.0 mL·min –1 , λ = 254.4 nm, t (major)=10.943, t (minor)= 12.549]; [ α ]25 D = 185 (c 0.16, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 7.31 (s, 1H), 7.18 – 7.08 (m, 2H), 7.04 (d, J = 7.9 Hz, 1H), 5.69(d, J = 1.6 Hz, 1H), 5.59 (dd, J = 9.0, 1.6 Hz, 1H), 5.21 – 4.99 (m, 2H),4.25 (qq, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com