Improved release dosage of febuxostat and preparation method of release dosage
A technology of febuxostat and slow-release clothing, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problem of high side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
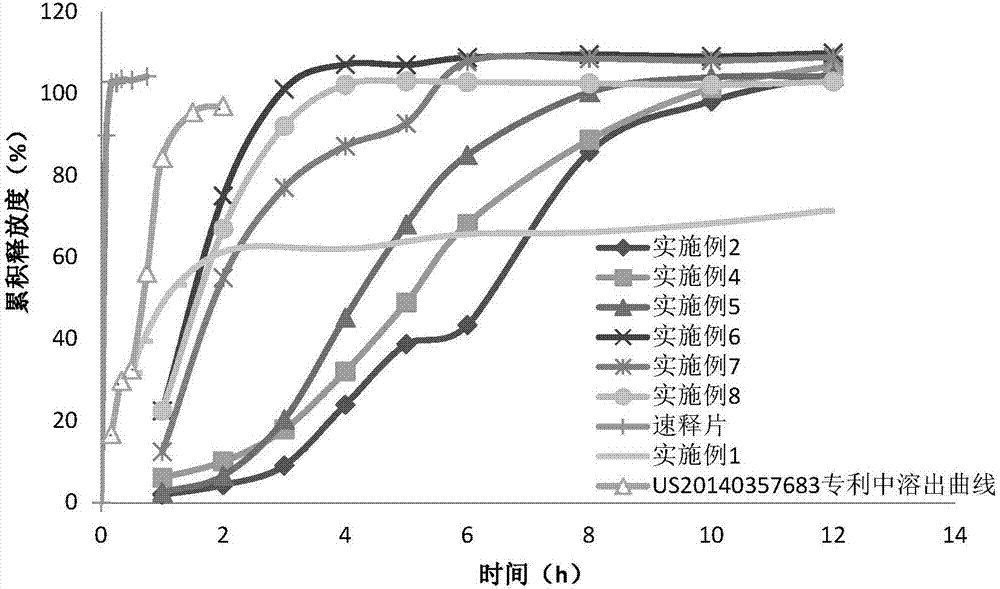
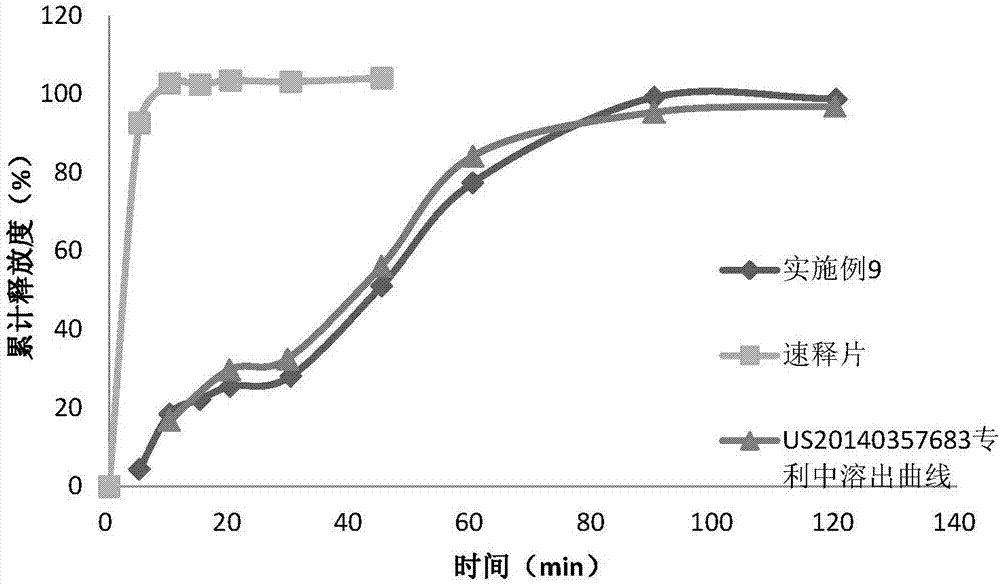
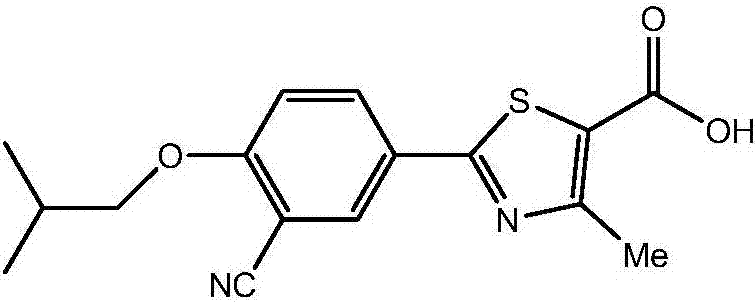
Image
Examples
Embodiment 1
[0087] Example 1 Formulation of febuxostat consisting of an immediate release portion of febuxostat and febuxostat sustained release pellets that release the drug slowly over a period of about 24 hours
[0088] The following composition is a single pulse and slow release drug delivery system in which a single capsule contains two types of febuxostat granules / pellets. One pulse consisted of immediate-release febuxostat granules containing a total of 20 mg of febuxostat, wherein the febuxostat was released immediately after ingestion by the patient. The remainder of the capsule contained controlled-release febuxostat pellets containing a total of 20 mg of febuxostat, which were released over an extended period of about 24 hours (immediately after ingestion by the patient). The immediate release febuxostat granules are prepared by dry granulation process, and its formulation composition is listed in Table 1 below. The composition of the controlled-release 24-hour pellets is list...
Embodiment 2
[0100] Example 2 The febuxostat preparation consisting of the febuxostat part for immediate release and the febuxostat sustained-release pellets for slow release of the drug over a period of about 12 hours under pH 6.8 conditions
[0101] The following composition is a single pulse and slow release drug delivery system under specific pH conditions, where a single capsule contains two types of febuxostat pellets. One pulse consisted of immediate-release febuxostat pellets containing a total of 12 mg of febuxostat, which was released immediately after ingestion by the patient. The remainder of the capsule contains a total of 28 mg of febuxostat under specific pH conditions (pH 6.8) slow-release febuxostat pellets, the febuxostat under pH 6.8 conditions for an extended period of about 12 hours Release (starts immediately after ingestion by the patient). The immediate-release febuxostat pellets were prepared by extrusion-spheronization process, and their formulation compositions ...
Embodiment 3
[0114] Embodiment 3 human body pharmacokinetic research
[0115] Taking the commercially available 40mg specification febuxostat tablet as the reference preparation, the human pharmacokinetic research was carried out together with the preparations obtained in Example 1 and Example 2, and the research results are shown in the following table:
[0116] Table 7. Pharmacokinetic parameters of different preparations
[0117] preparation
C max (ng / mL)
T max (h)
AUC 0→∞( ng / mL*h)
T C≥100μg / mL (h)
Fr(%)
Example 1
591±212.231
3.286±0.756
3522.18±981.16
10
44.54
Example 2
1067±788.339
3.188±2.267
6278.621±3186.562
12
79.38
1963.75±787.12
1.438±0.98
7908.342±3311.135
10
—
[0118] C max : maximum peak plasma concentration
[0119] T max : Maximum blood concentration time
[0120] AUC 0→∞ : drug-time curve area
[0121] T C≥100μg / mL : effective blood conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com