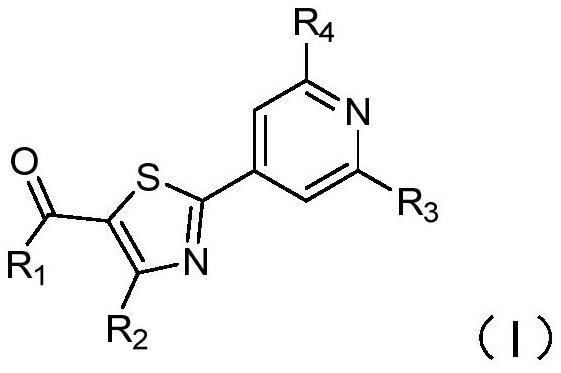
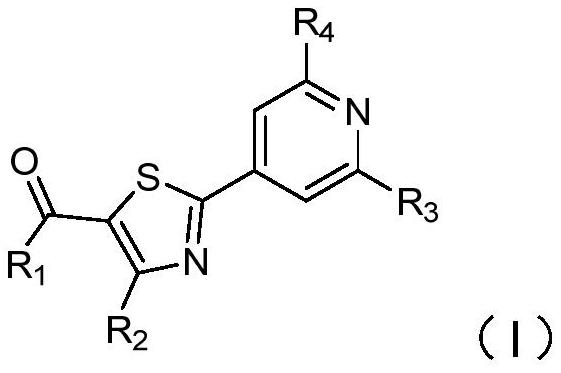
2-pyridyl thiazole derivative and application thereof
An alkyl and compound technology, applied in the field of 2-pyridyl thiazole derivatives, can solve problems such as lethal exfoliative dermatitis, injury, skin allergic reaction, liver and kidney function, etc., achieve prevention of hyperuricemia, excellent treatment and/or Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
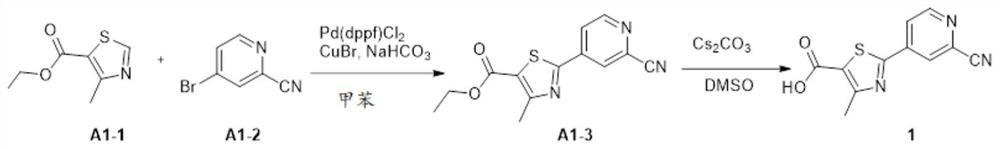
[0069] Compound 1: Synthesis of 2-(2-cyanopyridin-4-yl)-4-methylthiazole-5-carboxylic acid
[0070]
[0071] Step 1: Add ethyl 4-methylthiazole-5-carboxylate (3.2g, 21.1mmol), 4-bromopyridine-2-carbonitrile (3.0g, 16.3mmol), [1,1' - bis(diphenylphosphino)ferrocene]palladium dichloride (0.27g, 0.33mmol), sodium bicarbonate (3.4g, 34mmol), cuprous bromide (0.23g, 1.6mmol) and isobutyric acid Solution (0.04g), then add toluene (100ml), under nitrogen protection, heated to 110°C under stirring for 6 hours, cooled to room temperature, filtered the reaction solution, spin-dried, crude product with petroleum ether: ethyl acetate (10:1) Purified by column to obtain ethyl 2-(2-cyanopyridin-4-yl)-4-methylthiazole-5-carboxylate (A1-3) (3 g, 67.1%) as a white solid.
[0072] Step 2: Add ethyl 2-(2-cyanopyridin-4-yl)-4-methylthiazole-5-carboxylate (A1-3) (0.20g, 0.73mmol) to the reaction flask, then add cesium carbonate (0.24g, 0.73mmol) and dimethyl sulfoxide (5.0ml), heated to 90°C ...
Embodiment 2
[0077] Compound 2: Synthesis of 2-(2-cyanopyridin-4-yl)thiazole-5-carboxylic acid
[0078]
[0079] (compound 2)
[0080] In addition to the A1-1 (4-methylthiazole-5-ethyl carboxylate) in step 1
[0081] Replaced with ethyl thiazole-5-carboxylate Except that, according to the same steps and methods as in Example 1, compound 2 was synthesized.
[0082] LCMS (MS-ESI, m / z): (M+1) = 232.0
[0083] 1 H NMR (400MHz, DMSO-d 6,ppm): δ8.36(d,J=5.7Hz,1H),7.95(d,J=2.3Hz,1H),7.81(dd,J=5.7,2.2Hz,1H),7.46(s,1H) ,2.59(s,3H)
Embodiment 3
[0085] Compound 3: Synthesis of 2-(2-cyanopyridin-4-yl)-4-ethylthiazole-5-carboxylic acid
[0086]
[0087] Except for replacing A1-1 in step 1: ethyl 4-methylthiazole-5-carboxylate with ethyl 4-ethylthiazole-5-carboxylate, the compound was synthesized according to the same steps and methods as in Example 1 3.
[0088] LCMS (MS-ESI, m / z): (M+1) = 260.0
[0089] 1 H NMR (400MHz, DMSO-d 6 ,ppm): δ8.41(d,J=5.7Hz,1H),7.99(d,J=2.3Hz,1H),7.83(dd,J=5.7,2.2Hz,1H),7.46(s,1H) ,3.01(q,J=7.2Hz,2H),1.29(t,J=7.2Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com