Application of steroid saponin terpenoids and derivatives thereof in preparing medicine for treating purine metabolic disturbance diseases
A technology for steroidal saponins and abnormal metabolism, which can be used in steroids, bone diseases, drug combinations, etc., and can solve problems such as steroidal saponins that have not yet been found.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of salicin:
[0027] Taking the whole plant of Salix poplar grown in southern China as the raw material, the whole plant is dried in the shade and crushed, sieved, divided into coarse and fine powders, and loaded according to the fine powder, percolated with 10 times the amount of 30% ethanol, and the flow rate is controlled to 300ml / min. The leachate was filtered, concentrated to p=1.1, filtered with a 200-mesh filter cloth, and spray-dried to collect powder. The dry powder was degreased with petroleum ether and then air-dried. N-butanol was used as the solvent, the extraction temperature was 70°C, and the solvent dosage was 16 times the liquid-solid ratio for 4 hours. The obtained n-butanol extract was passed through a silica gel column, eluted with petroleum ether-ethyl acetate as a gradient of 100:0→10:90 to collect specific fractions, and then treated with a macroporous adsorption resin and chromatographed on a silica gel column for multiple ...
Embodiment 2
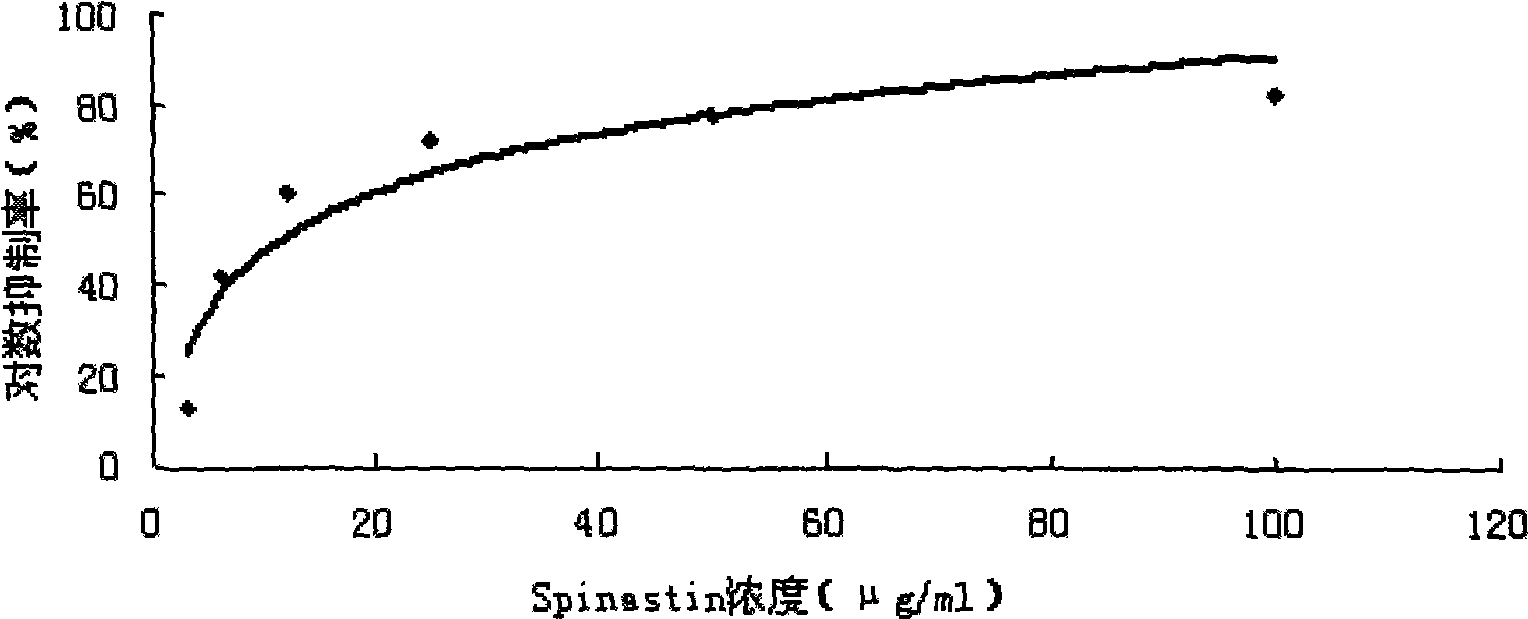
[0029] Fluorescence method to evaluate the effect of saponin Riparsaponin on xanthine oxidase in vitro, see figure 2 .
[0030] In this example, a 96-well fluorescence detection plate was used, and a positive control group, allopurinol, a negative control group, DMSO, and a test sample group were set up, and the total reaction system was 150 ul. The sample group to be tested has six concentrations, five parallel wells for each concentration, so that the final concentration is 3ug / ml, 6ug / ml, 12ug / ml, 25ug / ml, 50ug / ml, 100ug / ml, 1ul / well; positive; The control is 6 concentrations of allopurinol, the final concentration is 0.3, 0.6, 1.2, 2.4, 4.8, 9.6ug / ml, 1ul / well; the negative control DMSO, 1ul / well; use a fluorescence chemiluminescence detector at the excitation wave Measure the fluorescence value of each well at the emission wave of 380nm / 440nm, obtain the mean value, and calculate the inhibition rate and IC 50 value.
[0031] It can be seen from Table 2 that the saponi...
Embodiment 3
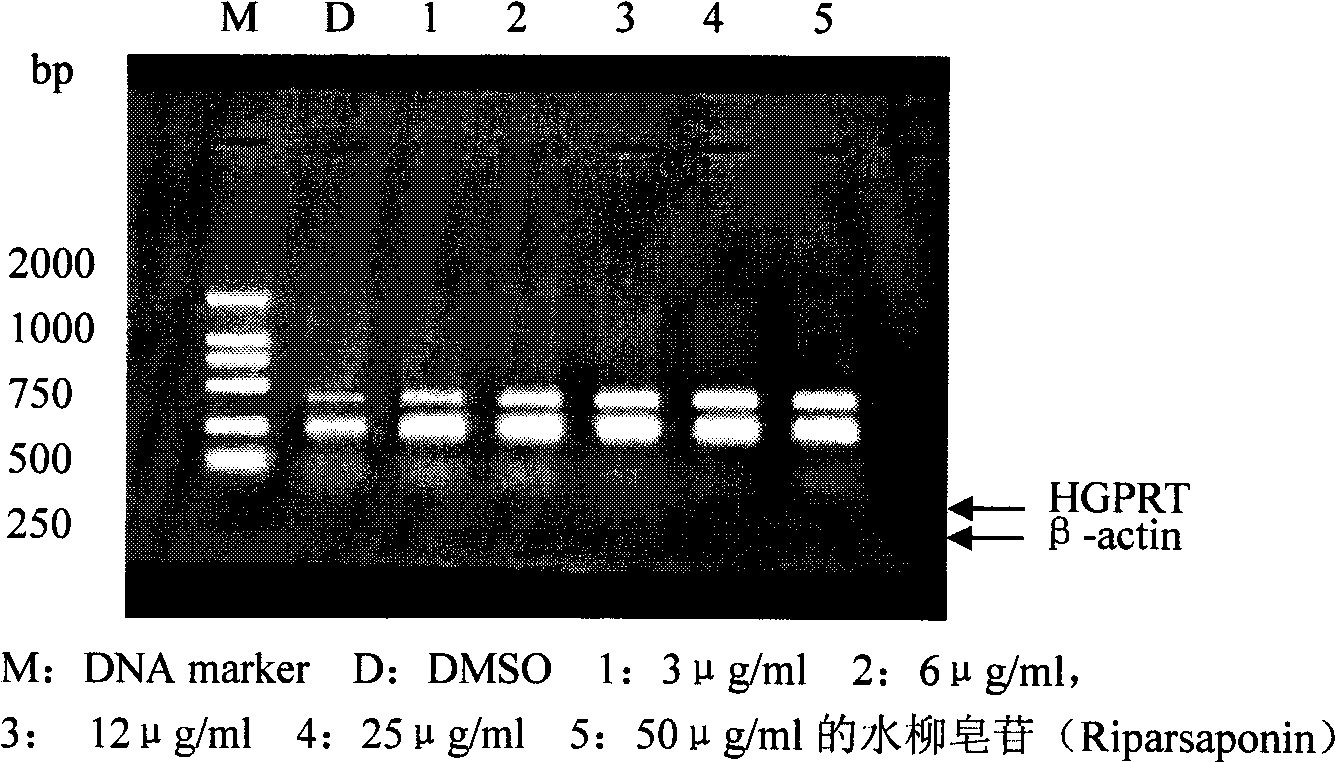
[0037] The effect of Riparsaponin on hypoxanthine-guanine phosphoribosyltransferase (HGPRT) was evaluated.
[0038] The ratio of HGPRT and β-actin PCR products was used to represent the HGPRT mRNA level. Compared with the control group, the HGPRT mRNA expression in the test sample group increased with the increase of the drug concentration, wherein the drug concentration was 12 μg / ml, 25 μg / ml. ml, the group rank of 50μg / ml was significantly different from the blank group (p image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com