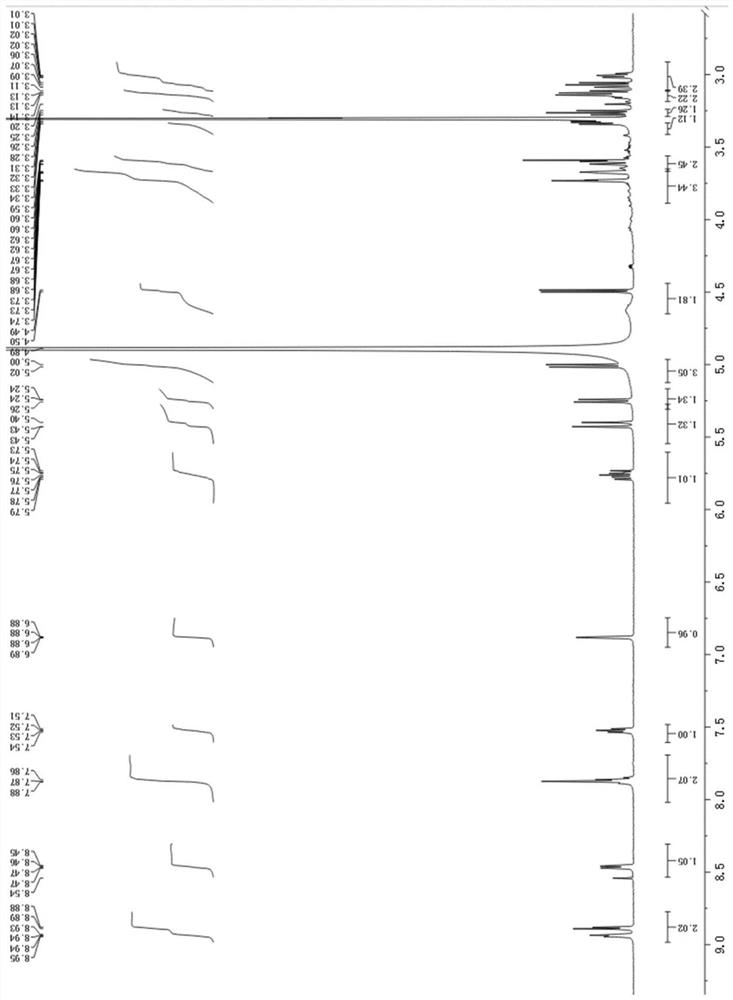
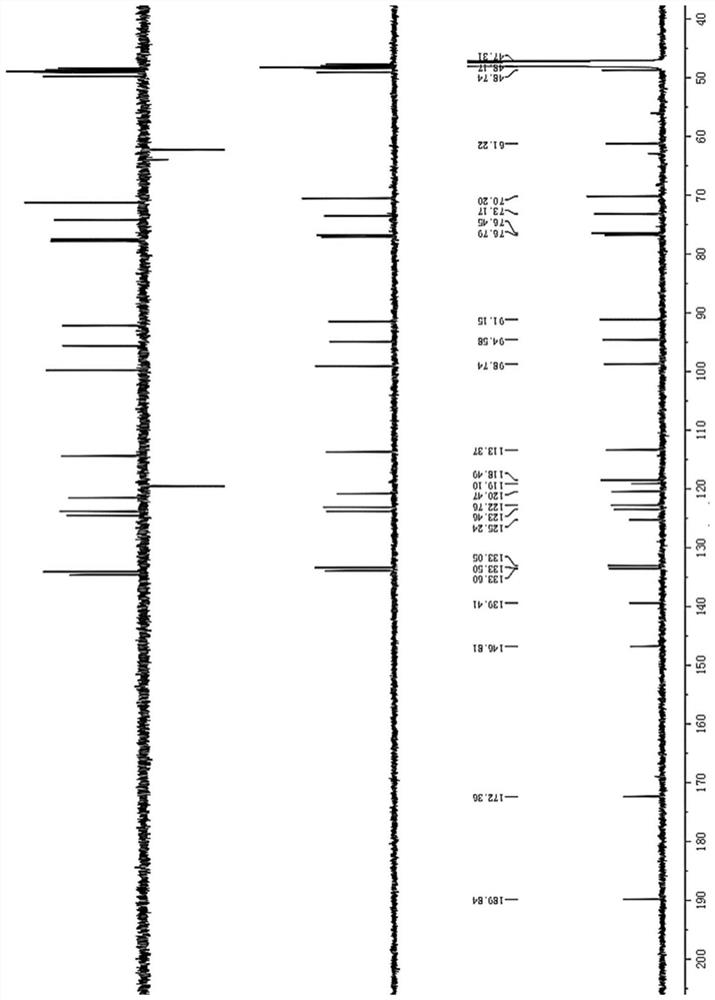
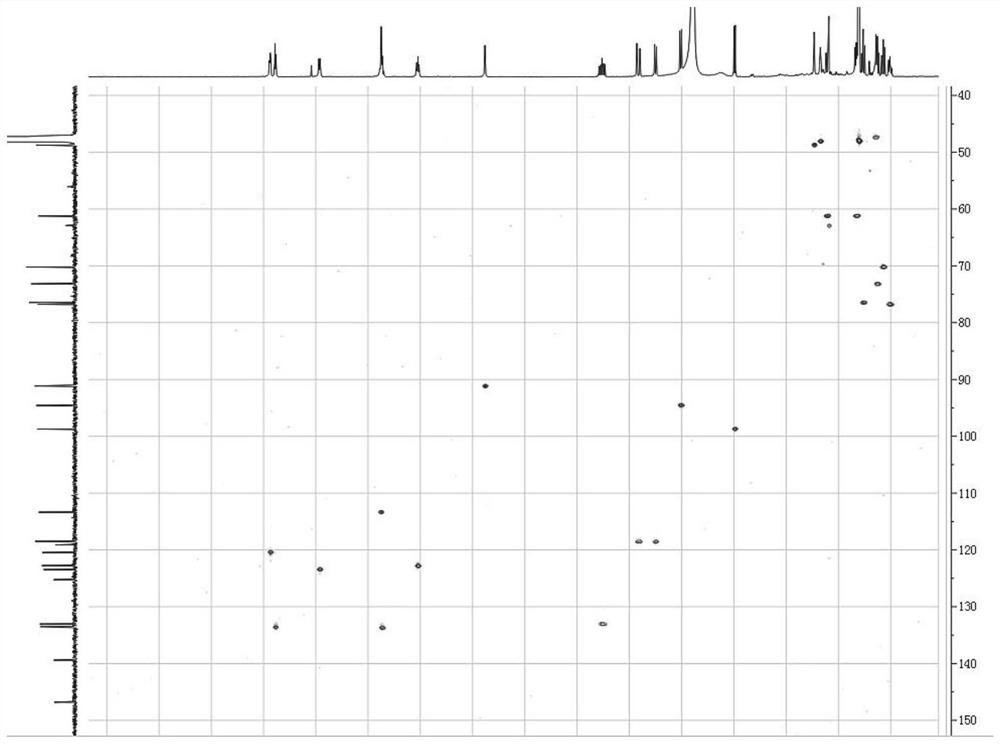
Novel isovimbin derivative derived from Japanese snakeroot as well as preparation method and application of novel isovimbin derivative
A technology of sardidines and derivatives, which is applied in the fields of medicine and chemistry, and can solve the problems of few active research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: The compound described in the claims and description of the invention - a kind of isosidine derivatives is obtained as follows:
[0029]The separation and purification process of the compound: the freshly picked Japanese snakeroot (Ophiorrhiza japonica) whole plant was dried, and 10kg of the dried Japanese snakeroot (Ophiorrhiza japonica) whole plant was taken, crushed and washed with 80% (v / v) methanol The aqueous solution was soaked 3 times (3×20L), each soaked for 2 days, the three extracts were combined, concentrated under reduced pressure, methanol was recovered, and the total extract was obtained. The total extract is completely dissolved with 0.5wt% HCl solution, adjusted to pH 2-3 with 1.85wt% HCl solution, extracted 3 times with an equal volume of ethyl acetate; the acidic water part is adjusted to pH 8 with 2.8wt% ammonia water ~9, and extracted three times with an equal volume of ethyl acetate. The remaining alkaline water layer was subjected to c...
Embodiment 2
[0039] Example 2: Xanthine oxidase inhibitory activity
[0040] Principle: Add different mass concentrations of raw materials, xanthine and xanthine oxidase into the reaction system. Under the catalysis of xanthine oxidase, the substrate xanthine is oxidized into uric acid. Uric acid has a characteristic absorption peak at 295nm. By measuring the reaction The production rate of product uric acid is used to determine the influence of raw materials on the in vitro activity of xanthine oxidase, and to calculate the IC 50 value.
[0041] 1. Solution preparation:
[0042] Phosphate buffer:
[0043] Take K 2 HPO 4 14.87g, KH 2 PO 4 1.99g, 0.1861g of EDTA-2Na (disodium ethylenediaminetetraacetic acid), dissolved in 500ml of water, and made into 0.2mol / L phosphate buffer (pH7.5).
[0044] Xanthine solution (substrate):
[0045] Weigh 1mg of xanthine and dissolve it with 1mL DMSO (dimethyl sulfoxide) to obtain a 1mg / mL mother solution. When using, take 400μL of the mother sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com