Method for preparing clinic-level CAR-T cell preparation by minicircle DNA transfection T cells
A cell preparation, -CAR-T technology, applied in the biological field, can solve problems such as no experimental confirmation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0085] The present invention is further illustrated below through specific examples, but not as a limitation of the present invention.
[0086] The technology for preparing clinical-grade PSCA-CAR-T cell preparations by transfecting T cells with microcircular DNA, the steps are as follows:
[0087] 1. Design and synthesis of PSCA-CAR gene sequence:
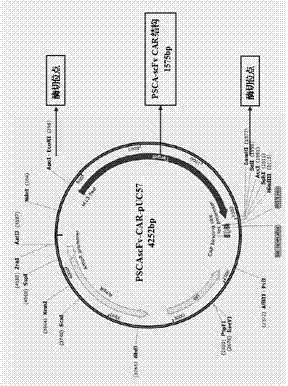
[0088] 1.1 Apply the biological software DNAstar8.0 to design the anti-PSCA Fab, VH and VL sequences, which are used to design the scFv sequence of CAR; design the signal peptide sequence IgGkappa to be placed in the front of the PSCA-scFv sequence; according to the CD8, CD28, CD137 and CD3ζ gene sequences are used to synthesize the framework structure of the third-generation CAR, and EcoR1 and BamH1 restriction sites are set at the front and rear ends of the CAR, respectively. According to the above design, the whole gene sequence of PSCA-CAR was synthesized (attached figure 1 ).
[0089] 1.2 Subcloning the PSCA-CAR gene seque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com