Application of dextromethorphan hydrobromide in the treatment of acute and chronic renal fibrosis
A technology for dextromethorphan hydrobromide and renal fibrosis, applied to medical preparations containing active ingredients, organic active ingredients, pharmaceutical formulas, etc., to prevent and inhibit the weakening of renal function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, configure dextromethorphan hydrobromide injection.
[0026] Dextromethorphan hydrobromide (molecular formula C 18 h 25 NO·HBr·H 2 O, referred to as DXM), white powder, purchased from Sigma-Aldrich Company. Take 100mg of DXM powder, dissolve it with normal saline to 10ml, and prepare a 10mg / ml mother solution. Before use, take 0.5ml, 1.5ml, and 3ml of the mother solution, dilute it with normal saline to 10ml, and prepare 0.5mg / ml, 1.5mg / ml, and 3mg / ml injections for low, medium, and Drug use in the high-dose group.
Embodiment 2
[0027] Example 2, test preparation.
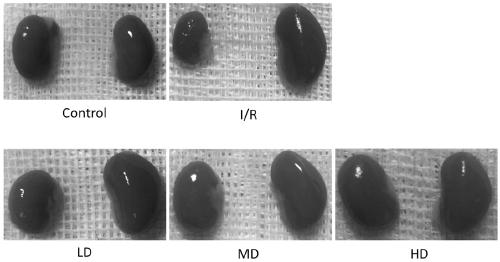
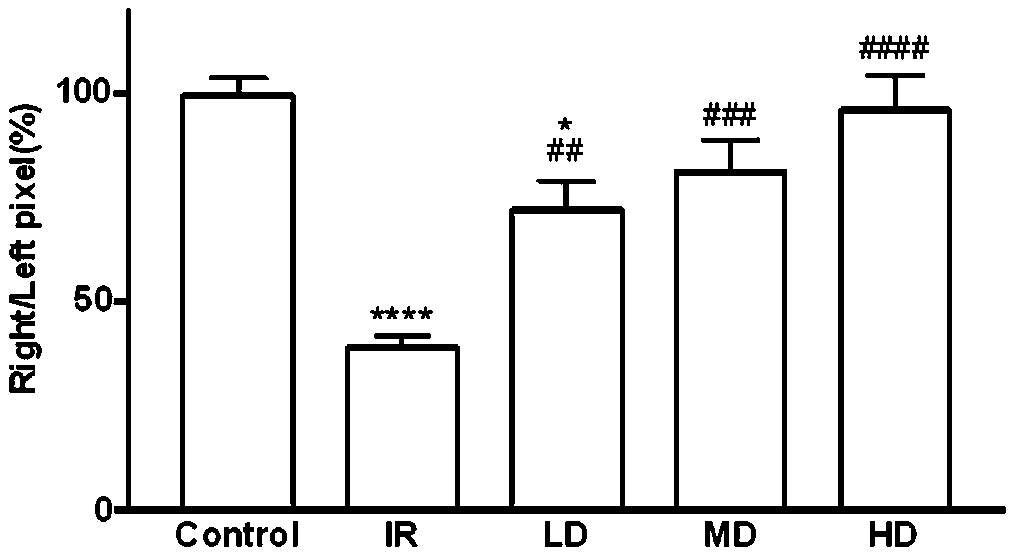
[0028] 8-week-old healthy male C57BL / 6 mice were purchased from the Experimental Animal Center of Zhejiang University, and the right renal artery was clamped and opened for 45 minutes to cause ischemia-reperfusion injury of the right kidney and lead to chronic renal fibrosis. Two weeks after ischemia / reperfusion, mice were randomly divided into 4 groups, sham operation group (Control), ischemia-reperfusion (IR) group, IR+low dose (5 mg / kg, LD) group, IR+medium dose (15 mg / kg, MD) group, IR+high dose (30 mg / kg, HD) group, 8 rats in each group. The sham operation group was given normal drinking water.
Embodiment 3
[0029] Example 3, verifying the effect of DXM on reducing serum creatinine in ischemic mice.
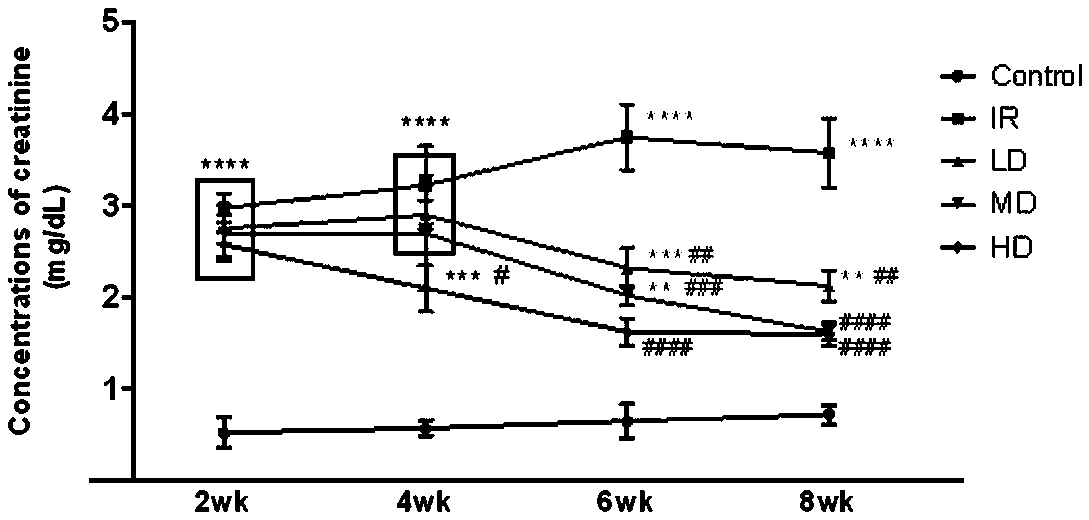
[0030] On the basis of Example 2, after ischemia, the mouse serum creatinine was measured every two weeks, and the results were significantly different using GraphPad Prism 7.0 for Two-way ANOVA analysis, the results are as follows figure 1 It was shown that the serum creatinine of renal ischemia mice before medication was significantly higher than that of the sham operation group; after 2 weeks (4wk) of medication, the serum creatinine of mice in the high-dose group decreased first, which was significantly lower than that of the non-medication group; after 4 weeks of medication (6wk) After 6 weeks (8wk) and 6 weeks (8wk), the high, medium and low doses all have a significant down-regulation effect on the serum creatinine value of mice, and there is a certain dose effect. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com