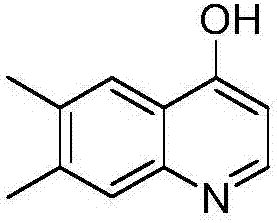
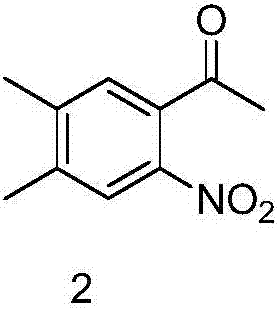
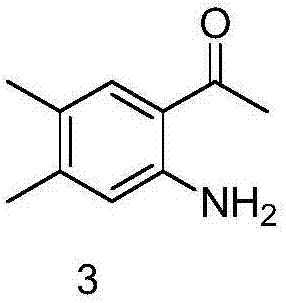
Preparation method of 6,7-dimethyl-4-hydroxyquinoline
A technology of hydroxyquinoline and dimethylphenyl, which is applied in the field of novel preparation of pharmaceutical intermediates and can solve problems such as difficulty in synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Synthesis of 1-(4,5-dimethyl-2-nitrophenyl)ethanone
[0020] Add 29g of 1-(3,4-dimethylphenyl)ethanone to 260ml of acetic acid, add 62g of concentrated nitric acid, stir overnight at room temperature, concentrate, then add ethyl acetate and water, separate, dry, concentrate, and the residue 31 g of 1-(4,5-dimethyl-2-nitrophenyl)ethanone was obtained by column separation.
[0021] (2) Synthesis of 1-(2-amino-4,5-dimethylphenyl)ethanone
[0022] Add 30g of 1-(4,5-dimethyl-2-nitrophenyl)ethanone to 240ml of anhydrous methanol, then add 4g of 10% palladium carbon, pass in hydrogen, stir at room temperature for 4 hours, filter, and collect The filtrate was concentrated to obtain 22 g of 1-(2-amino-4,5-dimethylphenyl)ethanone.
[0023] (3) Synthesis of 6,7-dimethyl-4-hydroxyquinoline
[0024] Add 12g of sodium to 230ml of absolute ethanol, then 20g of (7-methyl-imidazo[1,2-a]pyridin-2-yl)methanol and 32g of ethyl formate, heat and reflux and stir for 9 hours, concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com