Preparation of n-pentanal from low-butene reaction feed
A mixture, n-valeraldehyde technology, applied in the preparation of carbon-based compounds, the preparation of organic compounds, hydrocarbons and other directions, can solve problems such as expensive, expensive removal, and complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
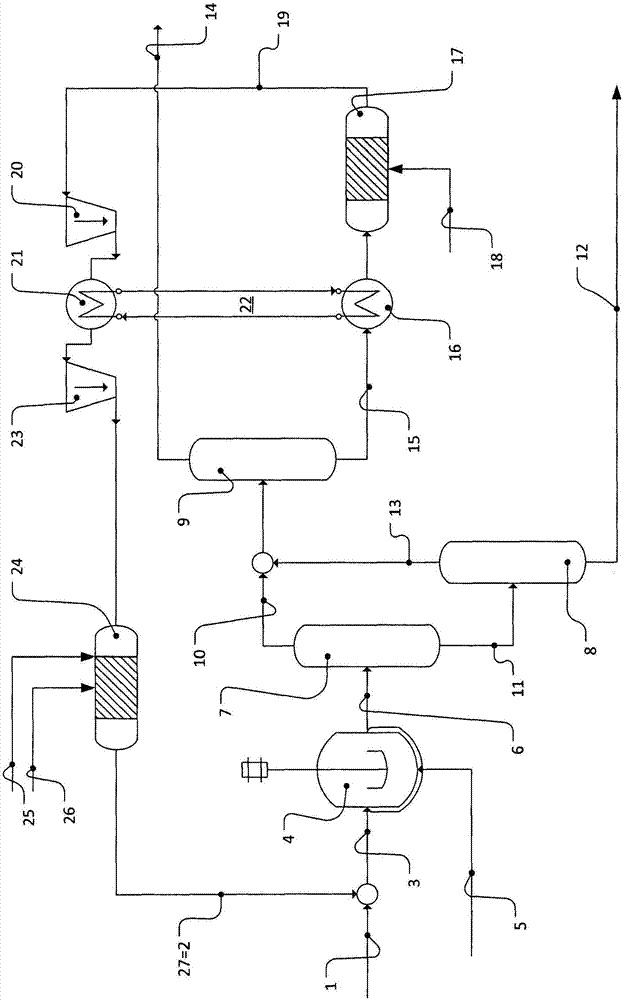
[0103] The basic idea of the inventive method is depicted in figure 1 middle. Feed mixture 1 obtained from outside the process and comprising mainly n-butanes and a balance of n-butenes is mixed with recycle 2 to provide feed 3 . This recycle originates from the process itself, which is further described later.
[0104] The feed 3 enters the hydroformylation 4 and reacts there with synthesis gas 5 , which is a mixture of carbon monoxide and hydrogen, in a conventional manner. The hydroformylation mixture 6 is withdrawn from the hydroformylation 4 and contains the desired n-valeraldehyde (formed from the reaction of n-butenes with synthesis gas), other by-products, unconverted n-butenes, especially Unconverted n-butane. The necessary separation of the homogeneous first catalyst system used in the hydroformylation 4 is not depicted here.
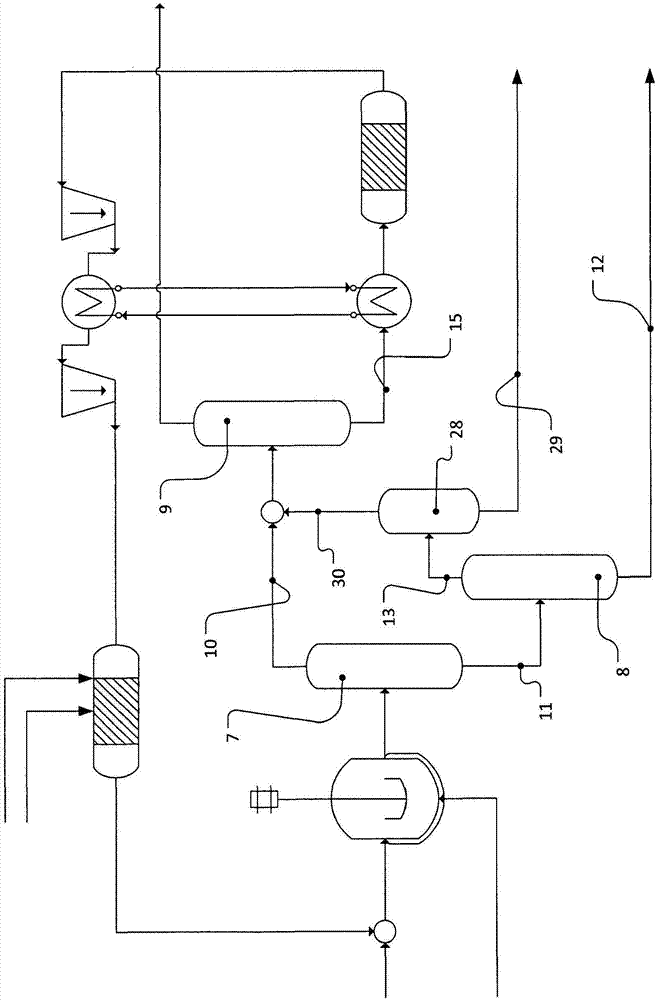
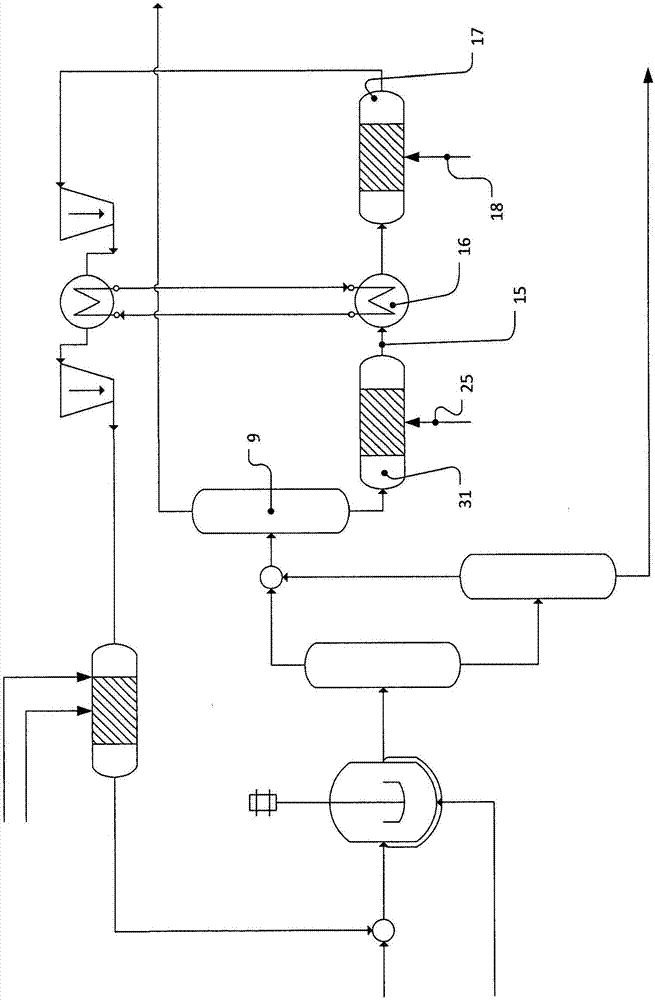
[0105] In a separation sequence comprising three distillation columns 7, 8, 9, the hydroformylation mixture is separated by distillatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com