Mononuclear iron complex and organic synthesis reaction using same
一种络合物、单核的技术,应用在铁有机化合物、有机化合物的制备、催化反应等方向,能够解决反应性低、强酸或路易斯酸高价贵金属催化剂、配位化合物稳定性低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
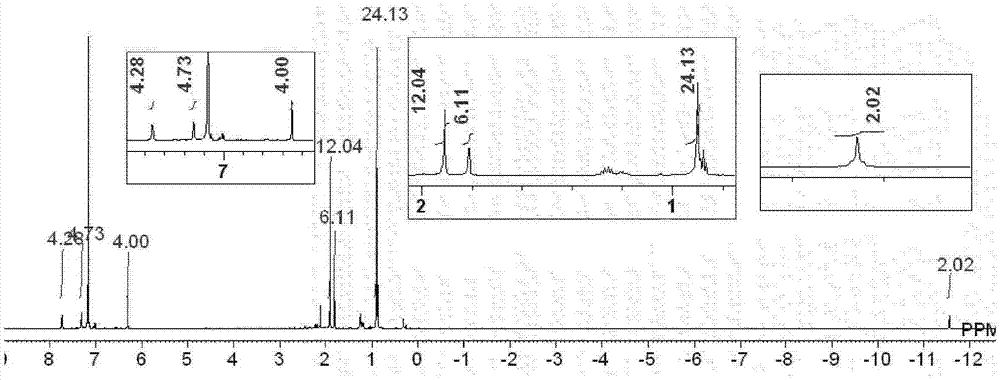
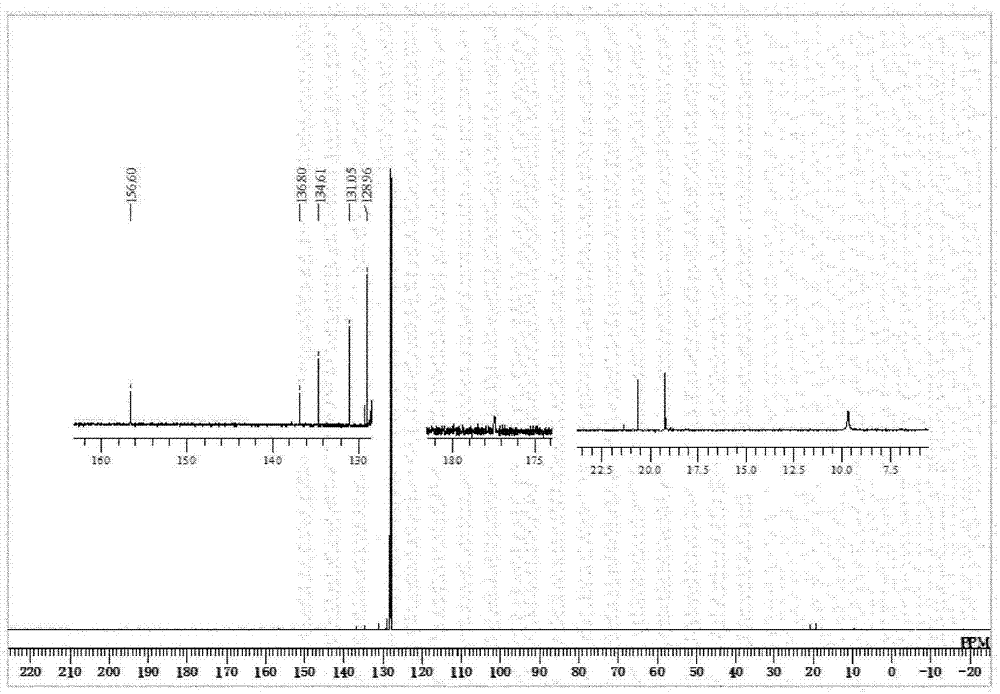
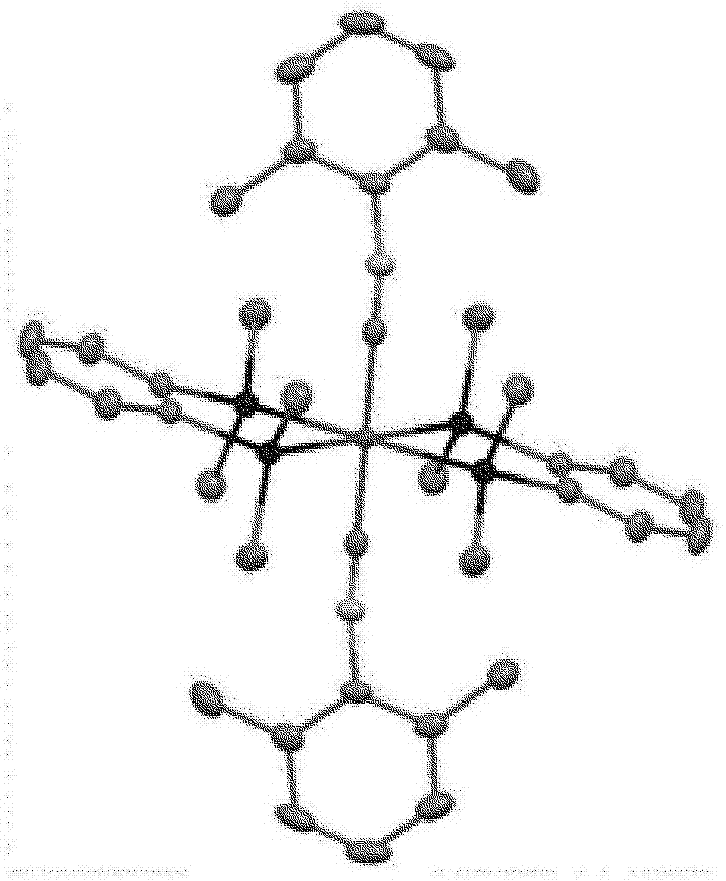
[0203] [Example 1] Synthesis of Iron Complex A
[0204] [chemical 13]
[0205]
[0206] (In the formula, Me means a methyl group.)
[0207] Under argon atmosphere, put (η 6 -1,3,5,7-Cyclooctatetraene)(η 4 -1,3,5,7-cyclooctatetraene)iron (0) complex (40mg, 0.15mmol) and 2,4,6-trimethylphenylisonitrile (66mg, 0.45mmol), to which Degassed and dehydrated hexane (40 mL) was added, followed by stirring at room temperature for 1 hour. Then, 1,2-bis(dimethylsilyl)benzene (64 mg, 0.33 mmol) was added, and after replacing the inside of the reaction vessel with a hydrogen atmosphere, a high-pressure mercury lamp (manufactured by USHIO Electric Co., Ltd., UM-453B- A (450W)) was stirred at room temperature for 48 hours while being irradiated with light. After the reaction was completed, it was dried under reduced pressure, and the obtained dried product was dissolved in toluene (10 mL), and a small amount of black insoluble matter produced as a by-product was removed by centrifugat...
Embodiment 2
[0213] [Example 2] Synthesis of Iron Complex B
[0214] [chemical 14]
[0215]
[0216] (In the formula, Me means a methyl group.)
[0217] Under argon atmosphere, put (η 6 -1,3,5,7-Cyclooctatetraene)(η 4 -1,3,5,7-cyclooctatetraene)iron (0) complex (100mg, 0.38mmol) and 2,6-dimethylphenylisonitrile (149mg, 1.14mmol) were added via Degassed and dehydrated hexane (60 mL) was stirred at room temperature for 1 hour. Then, 1,2-bis(dimethylsilyl)benzene (162 mg, 0.83 mmol) was added, and after replacing the inside of the reaction vessel with a hydrogen atmosphere, a high-pressure mercury lamp (manufactured by USHIO Electric Co., Ltd., UM-453B- A (450W)) was stirred at room temperature for 48 hours while being irradiated with light. After completion of the reaction, it was dried under reduced pressure, and the obtained dried product was dissolved in toluene (10 mL), and a small amount of black insoluble matter produced as a by-product was removed by centrifugation. Then, the...
Embodiment 3
[0222] [Example 3] Synthesis of Iron Complex C
[0223] [chemical 15]
[0224]
[0225] (In the formula, Me means a methyl group.)
[0226] Under argon atmosphere, put (η 6 -1,3,5,7-Cyclooctatetraene)(η 4 -1,3,5,7-cyclooctatetraene)iron (0) complex (50mg, 0.19mmol) and adamantyl isonitrile (92mg, 0.57mmol), to which was added degassed and dehydrated Alkane (50 mL), stirred at room temperature for 1 hour. Then, 1,2-bis(dimethylsilyl)benzene (80 mg, 0.42 mmol) was added, and after replacing the inside of the reaction vessel with a hydrogen atmosphere, a high-pressure mercury lamp (manufactured by USHIO Electric Co., Ltd., UM-453B- A (450W)) was stirred at room temperature for 48 hours while being irradiated with light. After completion of the reaction, it was dried under reduced pressure, and the obtained dried product was dissolved in toluene (5 mL), and a small amount of black insoluble matter produced as a by-product was removed by centrifugation. Then, the toluene s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com