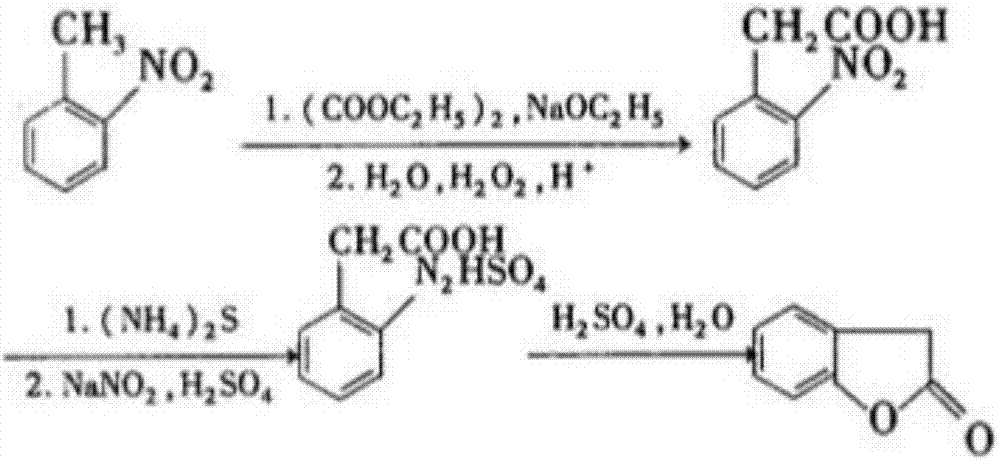
Synthesis process of azoxystrobin intermediate compound benzofuranone
A technology of benzofuranone and synthesis process, applied in directions such as organic chemistry, can solve problems such as long route, achieve the effects of simple operation, lower cost and overcoming long production process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] First put the raw material o-chlorophenylacetic acid into the synthesis kettle, then add catalyst and liquid caustic soda to the aforementioned synthesis kettle, heat up to 100°C for reaction; secondly, after the reaction of the aforementioned synthesis kettle is finished, cool down the temperature of the synthesis kettle to room temperature; then Filter the material in the synthesis kettle that was cooled to room temperature, and recover the catalyst by filtration; then transfer the filtered filtrate to the ring-closure kettle, and put the catalyst and toluene into the ring-closure kettle, raise the temperature to 100°C, and reflux the reaction 5 hours; then add water to the material after the aforementioned reflux to wash the impurities therein, transfer the aforementioned organic phase to a concentration tank for concentration; then after the aforementioned concentration of quantitative toluene, it is the toluene solution of benzofuranone; Finally, the benzofuranone i...
Embodiment 2
[0035] First put 800Kg of raw material o-chlorophenylacetic acid into a 2000L synthesis kettle, then add catalyst and liquid caustic soda to the aforementioned synthesis kettle, the concentration of liquid caustic soda is 40%, and heat up to 100°C for reaction. The catalyst is sodium ethoxide or quaternary ammonium salt One of them; secondly after the reaction of the aforementioned synthetic kettle is finished, the temperature of the synthetic kettle is cooled to room temperature; then the material in the aforementioned synthetic kettle cooled to room temperature is filtered, and the catalyst is recovered by filtration; then the aforementioned filtered filtrate Go to the ring-closing kettle, and put the catalyst and 300Kg toluene into the ring-closing kettle, raise the temperature to 100° C., and reflux for 5 hours. The catalyst is Raney Ni; The aforementioned organic phase is transferred to the concentration tank for concentration; then after the aforementioned concentration o...
Embodiment 3
[0038] First put 850Kg of raw material o-chlorophenylacetic acid into a 2000L synthesis kettle, then add catalyst and liquid caustic soda to the aforementioned synthesis kettle, the concentration of liquid caustic soda is 41%, heat up to 105°C for reaction, the catalyst is sodium ethoxide or quaternary ammonium salt One of them; secondly after the reaction of the aforementioned synthetic kettle is finished, the temperature of the synthetic kettle is cooled to room temperature; then the material in the aforementioned synthetic kettle cooled to room temperature is filtered, and the catalyst is recovered by filtration; then the aforementioned filtered filtrate Go to the ring-closing kettle, and put the catalyst and 350Kg toluene into the ring-closing kettle, raise the temperature to 105° C., and reflux for 6 hours. The catalyst is Raney Ni; The aforementioned organic phase is transferred to the concentration tank for concentration; then after the aforementioned concentration of qu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com