Double-layer tablet containing metformin hydrochloride and empagliflozin, and preparation method thereof
A metformin hydrochloride tablet, metformin hydrochloride technology, applied in the field of bilayer tablets containing metformin hydrochloride and empagliflozin and its preparation, drug compound preparation for treating and/or improving type 2 diabetes and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] According to the composition shown in Table 1, the double-layer tablet of the present invention is prepared, and the specific steps are:
[0105] 1) preparing metformin hydrochloride sheet-layer granules;
[0106] a) Sodium hydroxymethyl cellulose (50g) is dissolved in water to prepare a binder with a concentration of 10%;
[0107] b) Metformin hydrochloride (850g), hydroxypropyl methylcellulose K4M (400g), magnesium stearate (15g), and talcum powder (15g) were passed through a 100-mesh sieve respectively;
[0108] c) Add the sieved metformin hydrochloride and hydroxypropyl methylcellulose K4M to the SHK-10B multi-pot wet mixing rapid granulator and mix, wherein the stirring blade is 800rpm, the cutter is 2000rpm, and 120s;
[0109] d) Add the adhesive prepared in step a), and use a high-speed shear granulator to granulate, wherein the stirring blade is 800rpm, the cutter is 2000rpm, and 180s;
[0110] e) Use the UN1GLATT fluidized bed to dry the granules obtained in st...
Embodiment 2
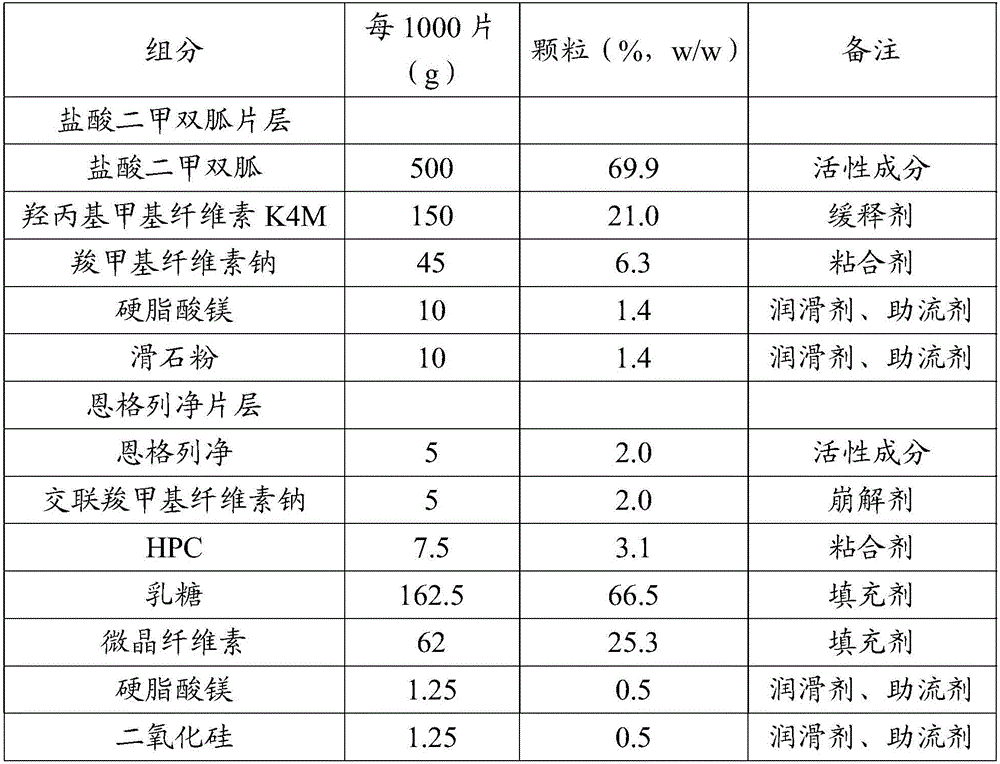
[0135] The composition of table 4 bilayer tablet
[0136]
[0137]
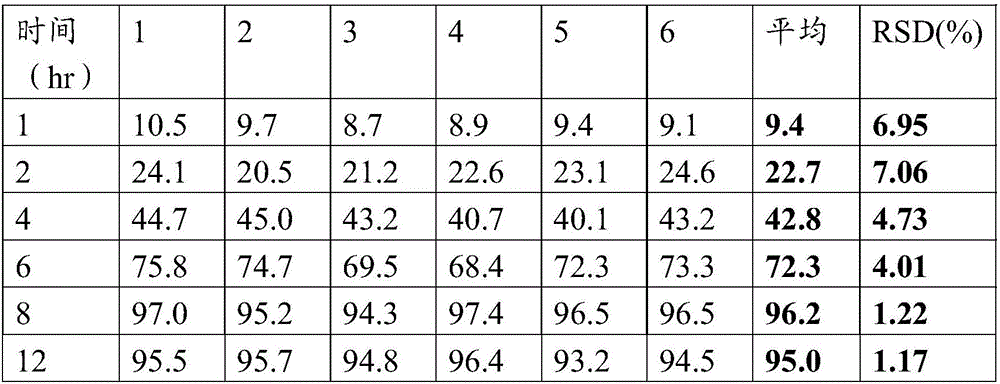
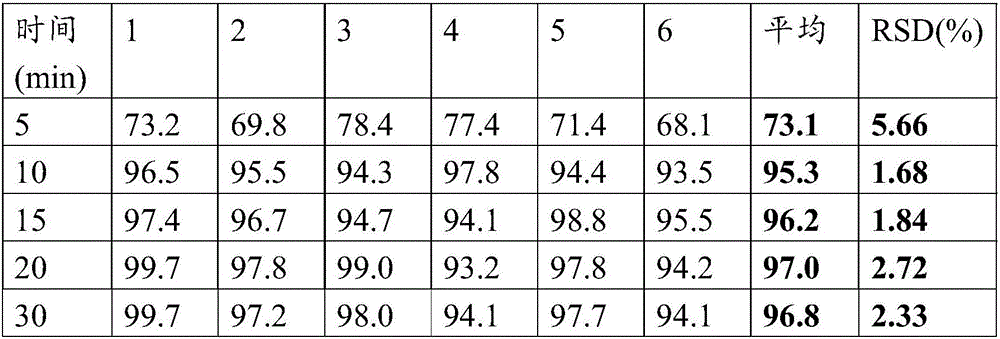
[0138] The preparation method is the same as in Example 1, and according to the solubility determination method described in Example 1, six double-layer tablets are randomly selected to measure the dissolution rate of metformin hydrochloride sheet and empagliflozin sheet respectively, and the results are shown in Table 5 and Table 5 respectively. Table 6 shows. It can be known from the table that the metformin hydrochloride slow-release layer releases steadily within 12 hours; the empagliflozin layer basically reaches full dissolution within 30 minutes.
[0139] Table 5 metformin hydrochloride sheet release test results
[0140]
[0141] Table 6 Empagliflozin tablet dissolution test results
[0142]
Embodiment 3
[0144] Table 7 Composition of bilayer tablet
[0145]
[0146] The preparation method is the same as in Example 1, and according to the solubility determination method described in Example 1, six double-layer tablets are randomly selected to measure the dissolution rate of metformin hydrochloride sheet and empagliflozin sheet respectively, and the results are shown in Table 8 and Table 8 respectively. Table 9 shows. It can be known from the table that the metformin hydrochloride slow-release layer releases steadily within 12 hours; the empagliflozin layer basically reaches full dissolution within 30 minutes.
[0147] Table 8 Metformin Hydrochloride Tablet Release Test Result
[0148]
[0149]
[0150] Table 9 Empagliflozin tablet dissolution test results
[0151]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com