nAChR-alpha1-ECD protein and preparation method thereof
A 1-ECD and protein technology, applied in the field of medical testing, can solve the problems of low yield, high cost, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
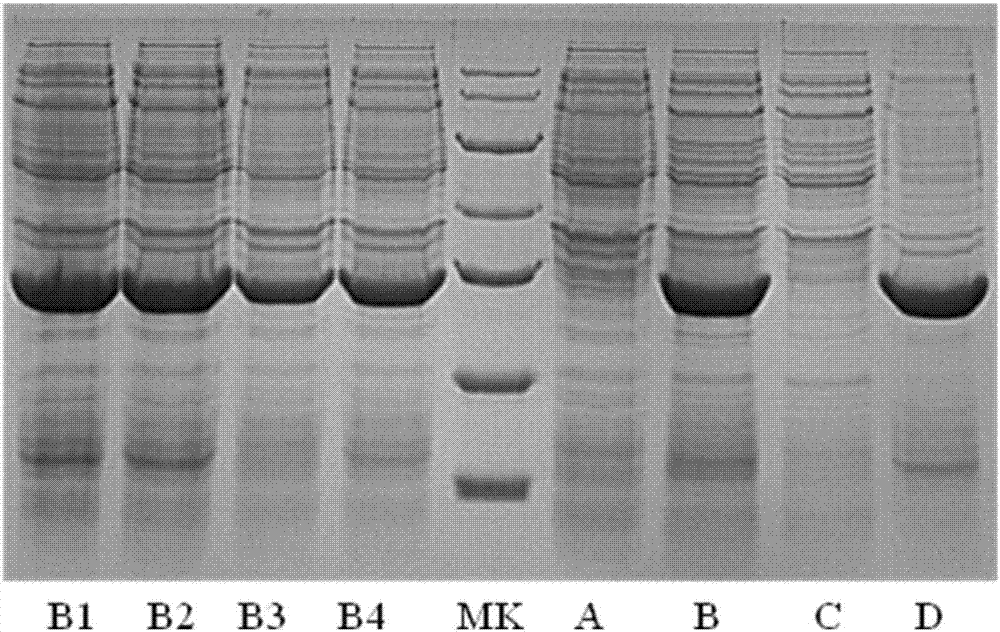
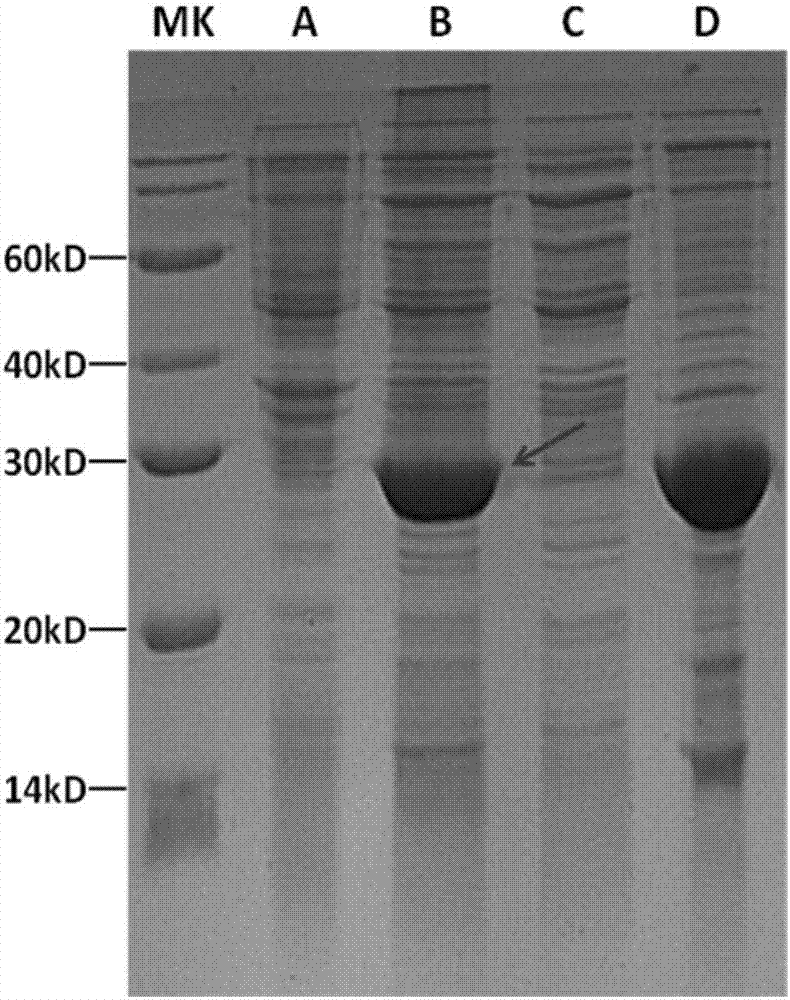
[0031] In order to make the technical means, creative features, goals and effects of the present invention easy to understand, the following examples will specifically illustrate the nAChR-α1-ECD protein and its preparation method of the present invention in conjunction with the accompanying drawings.
[0032] The reagents presented in the examples were purchased commercially unless the sources were indicated otherwise.
[0033] IPTG, isopropyl-β-D-thiogalactopyranoside.
[0034] LB medium, Luria-Bertani medium.
[0035] mM means mmol / L.
[0036] Tris is tris(hydroxymethyl)aminomethane buffer solution.
[0037] Gua-HCl is guanidine hydrochloride.
[0038] M is mol / L.
[0039] EDTA stands for ethylenediaminetetraacetic acid.
[0040] DTT is dithiothreitol.
[0041] WB is the abbreviation of Western blot.
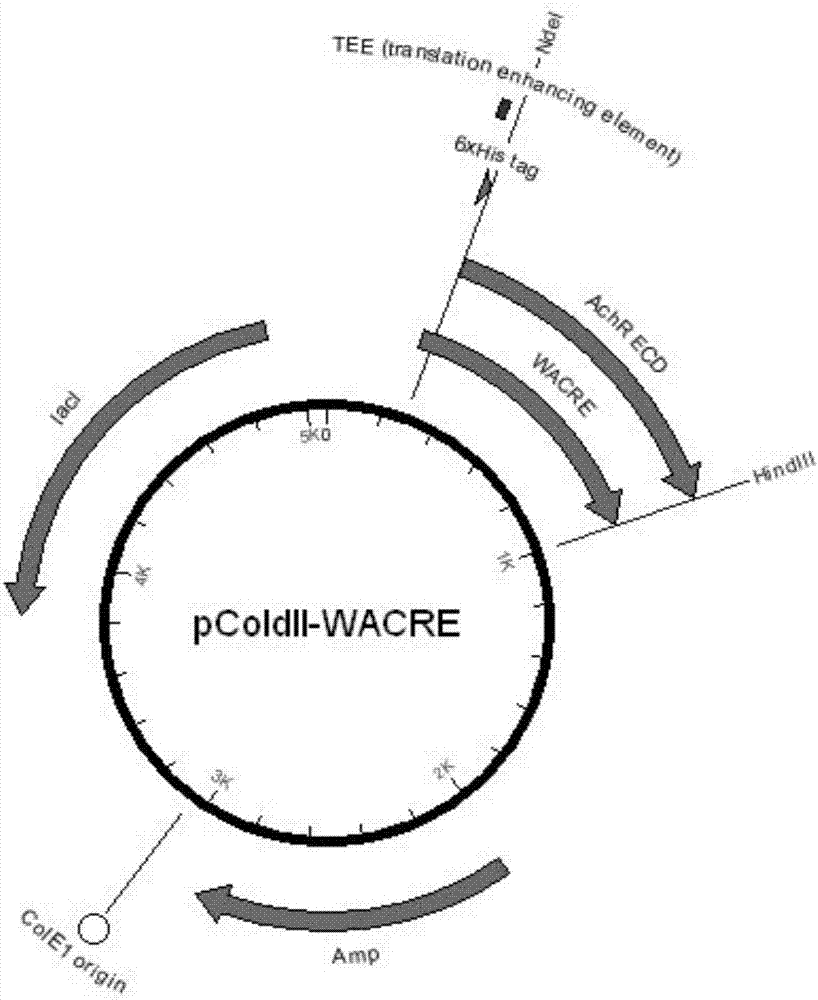
[0042] 1. Vector construction
[0043] Design the following SEQ ID 1 sequence on the basis of the protein of AChR-α1-ECD,
[0044] SEQ ID 1:
[0045] MNHKVHHHHHHM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com