Core-shell-structured oxide supported Pt catalyst as well as preparation method and application of Pt catalyst
A technology of core-shell structure and catalyst, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc. Improvement and other issues, to achieve the effects of low load, improved performance, and excellent low-temperature denitrification performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
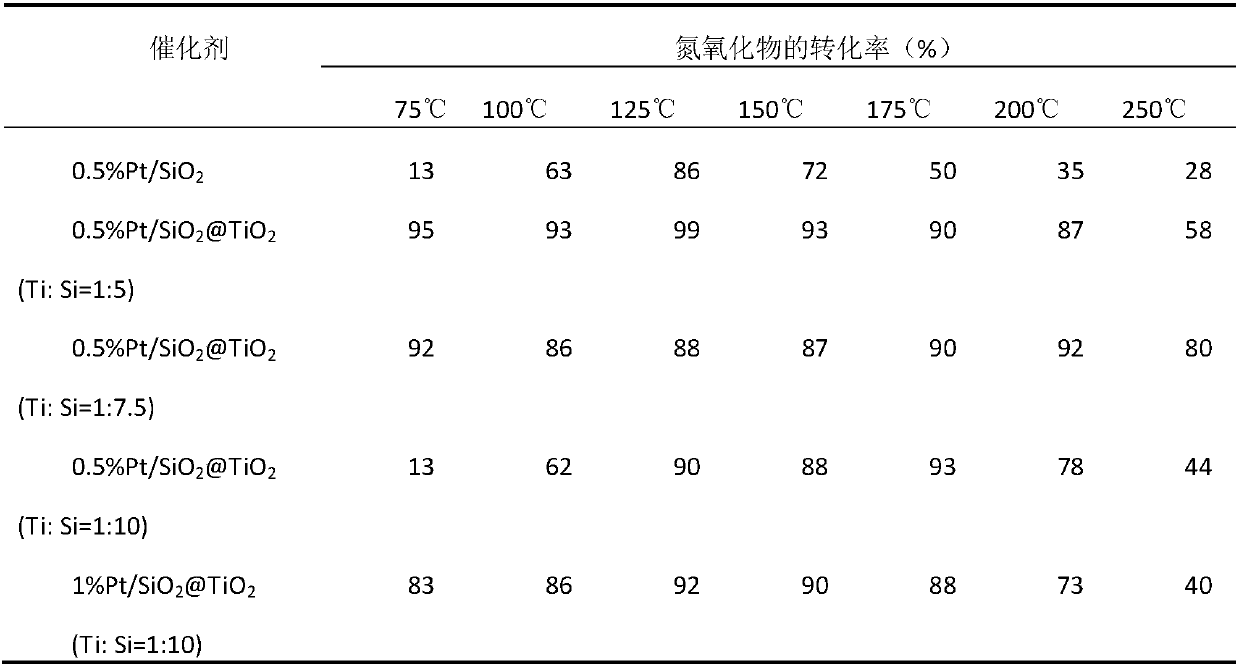
[0022] Example 1: 0.5% Pt / SiO 2 @TiO 2 Preparation of (Ti:Si=1:5) catalyst
[0023] a) Stir and mix 145mL ethanol, 95mL ammonia water and 20mL deionized water to obtain a mixed solution, then add 50mmol tetraethylorthosilicate to the mixed solution, heat to 50°C, reflux in a water bath for 2h, cool to room temperature, and then dissolve the reaction solution Perform centrifugal filtration and dry at 120°C for 24 hours to obtain spherical SiO 2 ;
[0024] b) 250mL ethanol and 10mmol tetrabutyl titanate were ultrasonically mixed to obtain a mixed solution, and the spherical SiO obtained in step (a) 2 Add it into the mixed solution, stir and sonicate for 30 minutes, and record it as mixed solution A; stir and mix 50mL ethanol and 250mL deionized water, and record it as solution B.
[0025] c) The solution B obtained in the step (b) is added dropwise to the mixed solution A, and the stirring reaction is continued for 12 hours after the dropwise addition is completed. The solu...
Embodiment 2
[0028] Example 2: 0.5% Pt / SiO 2 @TiO 2 Preparation of (Ti:Si=1:7.5) catalyst
[0029] a) Stir and mix 145mL ethanol, 95mL ammonia water and 20mL deionized water to obtain a mixed solution, then add 50mmol tetraethylorthosilicate to the mixed solution, heat to 70°C, reflux in a water bath for 4h, cool to room temperature, and then dissolve the reaction solution Perform centrifugal filtration and dry at 120°C for 12 hours to obtain spherical SiO 2 ;
[0030] b) 200mL of ethanol and 6.67mmol of tetrabutyl titanate were ultrasonically mixed to obtain a mixed solution, and the spherical SiO obtained in step (a) 2 Add it into the mixed solution, stir and sonicate for 30 minutes, and record it as mixed solution A; stir and mix 50mL ethanol and 200mL deionized water, and record it as solution B;
[0031] c) The solution B obtained in step (b) was added dropwise to the mixed solution A, and after the dropwise addition was completed, the stirring reaction was continued for 8 hours. ...
Embodiment 3
[0034] Example 3: 0.5% Pt / SiO 2 @TiO 2 Preparation of (Ti:Si=1:10) catalyst
[0035] a) Stir and mix 145mL ethanol, 95mL ammonia water and 20mL deionized water to obtain a mixed solution, then add 50mmol tetraethyl orthosilicate to the mixed solution, heat to 60°C, reflux in a water bath for 3h, cool to room temperature, and then dissolve the reaction solution Perform centrifugal filtration and dry at 120°C for 12 hours to obtain spherical SiO 2 ;
[0036] b) 180mL ethanol and 5.0mmol tetrabutyl titanate were ultrasonically mixed to obtain a mixed solution, and the spherical SiO obtained in step (a) 2 Add it into the mixed solution, stir and sonicate for 30 minutes, and record it as mixed solution A; stir and mix 50mL ethanol and 250mL deionized water, and record it as solution B;
[0037] c) The solution B obtained in the step (b) is added dropwise to the mixed solution A, and the stirring reaction is continued for 10 h after the dropwise addition is completed. The solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com