Film strip for typing neuromyelitis optica pathopoiesis autoantibodies
A technology for neuromyelitis optica and autoantibodies, which is applied in the medical field and can solve problems such as rough identification methods and inability to meet clinical precision medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] see Figure 1-4 , a membrane strip for autoantibody typing of neuromyelitis optica, comprising NMO-IgG typing membrane strip 1, small peptide A, small peptide C and small peptide E;
[0031] Based on the dominant epitope sequence of the outer antigen of AQP4 cell membrane, three small peptides were synthesized, namely:
[0032] Small peptide A, the amino acid sequence of small peptide A is TINWGGTEKPLPVDMV from N-terminal to C-terminal;
[0033] Small peptide C, the amino acid sequence of small peptide C is CVTPPSVVGGLGVTTVHGNLTAG from N-terminal to C-terminal;
[0034] Small peptide E, the amino acid sequence of small peptide E is INYTGASMNPARSFGPAVIMGNWENHW from N-terminal to C-terminal;
[0035] The preparation method of the NMO-IgG typing membrane strip 1 comprises the following steps:
[0036] 1), first use polyacrylamide gel electrophoresis to separate the three small peptides in the same lane by using the difference in molecular weight, and the three small pe...
experiment example 1
[0042] Utilize the NMO-IgG typing film strip 1 of embodiment 1 to detect two patient serums (being respectively patient A and B) who have been diagnosed as NMO clinically, the test results are shown in figure 2 , it was found that the serum of patient A in the left swimming lane contained NMO-IgG for three small peptides, but the content of NMO-IgG for small peptide E was less. The patient B serum in the right lane contains both NMO-IgG for small peptide A and small peptide C, and NMO-IgG for small peptide E was not detected.
experiment example 2
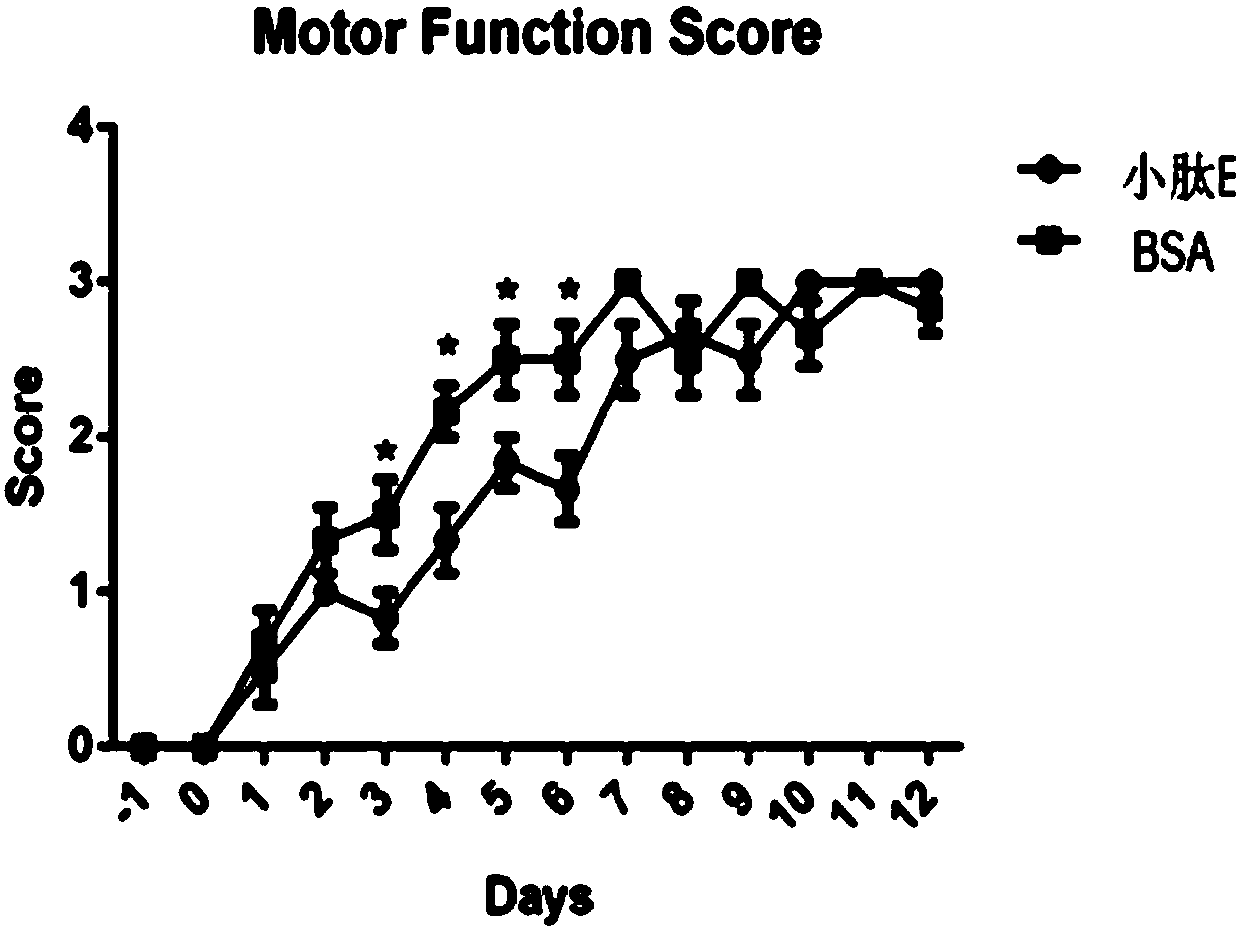
[0044] According to the test results of Experimental Example 1, two groups of NMO animal model tests were carried out on patient B serum respectively, and the reliability of the detection of NMO-IgG typing membrane strip 1 was verified. image 3 In the middle, the left picture shows that patient B serum was co-incubated with BSA, and then injected into the central nervous system of rats after co-incubation with small peptide E. Through behavioral testing, it was found that small peptide E could not effectively reduce NMO-IgG in the patient's serum toxicity. Subsequently, patient B serum was co-incubated with BSA, and then injected into the central nervous system of rats after co-incubation with small peptide A and small peptide C. Through behavioral testing, it was found that small peptide A and small peptide C could significantly reduce the serum levels of patients. Toxicity of NMO-IgG. This experimental result is completely consistent with the detection result of NMO-IgG typi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com