Application of D-xylose product to preparation of UTI (urinary tract infection) preventing and treating drug
A technology for urinary system infection and xylose is applied in the application field of monosaccharide xylose products in the preparation of drugs for preventing and treating urinary system infection, and achieves the effects of excellent processing characteristics and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
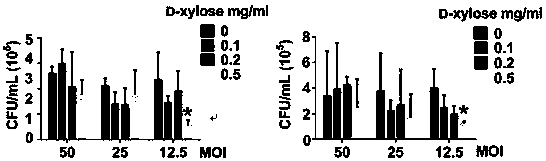
[0028] Effect of D-xylose on CFT073 infecting bladder epithelial cell 5637 and kidney epithelial cell 786-O
[0029] 1) Take a little bacterial solution from the strain preservation tube, streak and inoculate it on a Luria-Bertani solid plate, cultivate overnight at 37°C.
[0030] 2) Pick a monoclonal colony from the LB solid plate, inoculate it in 5 ml LB liquid medium, and culture overnight at 37°C.
[0031] 3) Take 1 ml of the bacterial solution into a 1.5 ml centrifuge tube, centrifuge at 6000 rpm for 5 minutes to collect the bacterial cells, discard the supernatant, wash with PBS, and resuspend the bacterial cells with the corresponding cell culture medium.
[0032] 4) Set different bacterial MOI values and different D-xylose concentrations to incubate with 5637 and 786-O cells for 2 hours.
[0033] 5) Discard the culture supernatant, wash 5 times with PBS, lyse with 0.2% Triton X-100 for 10 minutes, and collect the lysate.
[0034] 6) The above lysate was serially di...
Embodiment 2
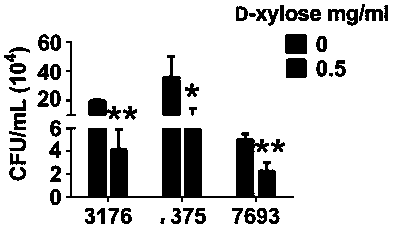
[0037]The role of D-xylose in the invasion of bladder epithelial cells 5637 by UPEC clinical isolate
[0038] 1) Take a little bacterial solution from the strain preservation tube, streak and inoculate it on LB solid plate, culture overnight at 37°C.
[0039] 2) Pick a monoclonal colony from the solid plate, inoculate it in 5 ml LB liquid medium, and culture overnight at 37°C.
[0040] 3) Take 1ml of the bacterial solution into a 1.5 mL centrifuge tube, centrifuge at 6000 rpm for 5 minutes to collect the bacterial cells, discard the supernatant, wash with PBS, and resuspend the bacterial cells with the corresponding cell culture medium.
[0041] 4) 5637 cells were co-incubated with 0.5 mg / ml D-xylose and different UPEC clinical isolates for 2 hours.
[0042] 5) The culture supernatant was discarded, washed 5 times with PBS, lysed with 0.2% Triton X-100 for 10 minutes, and the lysate was collected.
[0043] 6) The above lysate was serially diluted, spread on a non-resistant L...
Embodiment 3
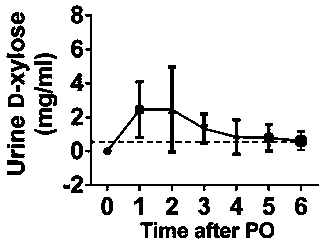
[0046] D-xylose content in urine within a certain period of time after administration of D-xylose in C57BL / 6J mice
[0047] Select C57BL / 6J female mice aged 6-8 weeks, give 100 mg / kg D-xylose orally, collect urine within 0-6 hours, and measure D in urine by phloroglucinol color development -xylose content.
[0048] The results showed that the D-xylose content in urine reached the highest within 1-2 hours after gavage, and D-xylose was basically excreted with urine after 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com