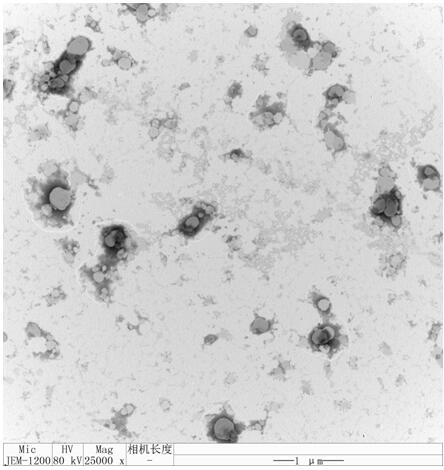
A kind of doxycycline controlled-release quaternary ammonium salt chitosan-liposome composite nanoparticle with pH responsiveness and preparation method thereof
A quaternary ammonium salt chitosan and doxycycline technology, applied in the field of biomedicine, can solve the problems of difficult to completely remove the infected site, increase resistance immune attack, threaten human health, etc., and achieve good development and application potential and manufacturing cost. Inexpensive, release-promoting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] 2. Preparation of liposomes: Weigh egg yolk lecithin (SPC), add chloroform and absolute ethanol to dissolve, the mass volume ratio of the mass of egg yolk lecithin, the volume of chloroform and the volume of absolute ethanol is 10 :1:1-20:1:1. Heat treatment in a water bath at 35°C for 1 h, so that the lecithin forms a uniform film on the inner wall of the flask. Then add the pH 7.4 PBS buffer solution containing sodium cholate and doxycycline at a mass ratio of 5:1, rotate and stir in a water bath at 55°C for 30 min, and obtain liposome nanoparticles loaded with doxycycline after ultrasonication. particle suspension.
[0027] 3. Preparation of pH-responsive doxycycline controlled-release quaternary ammonium chitosan-liposome composite nanoparticles: prepare 0.5 mg / mL-2 mg / mL of the quaternary ammonium chitosan solution, slowly drop In the liposome nanoparticle suspension of described entrapped doxycycline, stir while adding dropwise, the volume ratio of described quat...
Embodiment 1
[0030] The doxycycline controlled-release quaternary ammonium salt chitosan-liposome composite nanoparticle of the present embodiment is prepared by the following preparation method:
[0031] 1. Weigh 2 g of chitosan powder with a molecular weight of 15 kDa, add 6 mL of 15% (m / v) NaOH, and swell at room temperature for 10 min in a flat-bottomed flask; then add 20 mL of dimethyl sulfoxide and 6 mL of methyl iodide, React at 60°C for 1 h. After 1 h, 3 mL of NaOH and 3 mL of methyl iodide were added respectively, and the reaction was continued for 1 h. After the reaction was completed, absolute ethanol was added to form a white precipitate to terminate the reaction, dialyzed for three days, and freeze-dried. That is, water-soluble quaternary ammonium salt chitosan is obtained.
[0032] 2. Preparation of liposomes: Weigh 70 mg egg yolk lecithin (SPC), add 5 ml chloroform and 5 ml absolute ethanol to dissolve. Heat treatment in a water bath at 35°C for 1 h, so that the lecithin ...
Embodiment 2
[0035] The doxycycline controlled-release quaternary ammonium salt chitosan-liposome composite nanoparticle of the present embodiment is prepared by the following preparation method:
[0036] 1. Weigh 2 g of chitosan powder with a molecular weight of 150 kDa, add 12 mL of 15% NaOH, swell in a flat-bottomed flask at room temperature for 10 min, add 40 mL of dimethyl sulfoxide and 12 mL of methyl iodide, and react at 60 °C for 1 h. After 1 h, 6 mL of NaOH and 6 mL of iodomethane were added, and the reaction was continued for 1 h. After the reaction was completed, absolute ethanol was added to form a white precipitate to terminate the reaction, dialyzed for three days, and freeze-dried. That is, water-soluble quaternary ammonium salt chitosan is obtained.
[0037] 2. The preparation method of the liposome nanoparticle suspension loaded with doxycycline is the same as that in Example 1.
[0038] 3. Prepare 2 mg / mL of the quaternary ammonium chitosan solution, slowly add it into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com