Preparation method of edoxaban intermediate
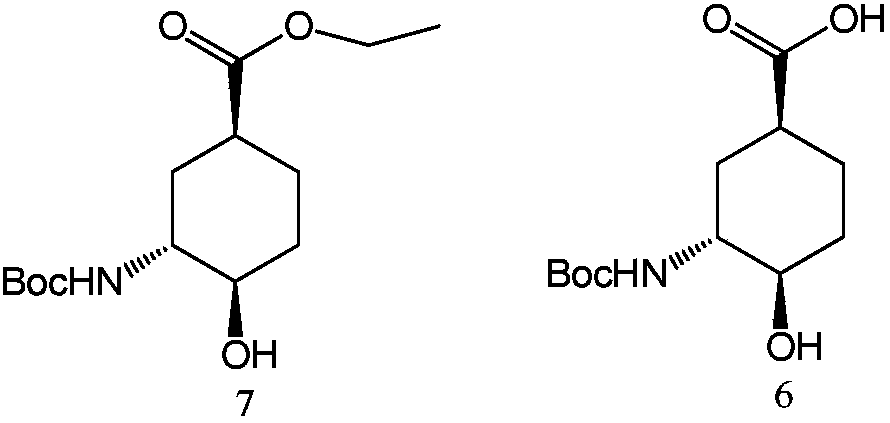
A technology for edoxaban and intermediates, applied in the field of preparation of edoxaban intermediates, can solve the problems of being unsuitable for industrial production, expensive, and the production cost of the compound of formula 6, etc., and achieve large-scale industrial production, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
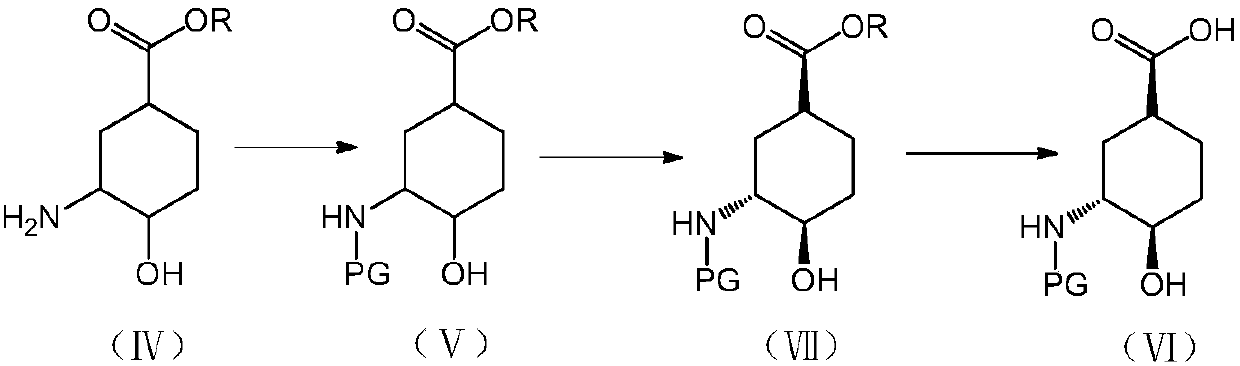
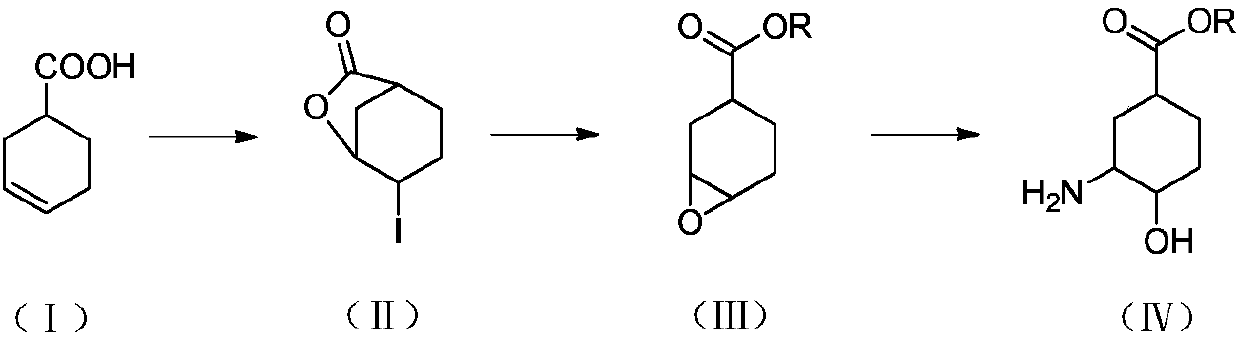
[0035] Embodiment 1, the preparation of (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxyl-cyclohexanecarboxylic acid
[0036] The preparation route is as follows:
[0037]
[0038] (1) Preparation of 4-iodo-3-cyclohexylcarboxylate
[0039] Dissolve 200g of cyclohexene-1-carboxylic acid (1) in 600mL of dichloromethane, add 200g of sodium bicarbonate, 350g of potassium iodide, and 500mL of water under ice-cooling. After 10 minutes, warm up to room temperature and add 500g of iodine. After 2 hours, , adding sodium thiosulfate aqueous solution (2N, 300mL), after half an hour, separate the layers, extract the aqueous phase once with 500mL dichloromethane, combine the organic phases, dry, and spin dry to obtain 460g white solid (2).
[0040] (2) Preparation of 7-oxabicyclo[4.1.0]heptane-3-carboxylic acid methyl ester
[0041] Add 30 g of 4-iodo-3-cyclohexanecarboxylic acid lactone into 120 mL of methanol, stir at room temperature for 10 minutes, add sodium hydroxide (50 mL, 2....
Embodiment 2
[0049] Embodiment 2, the screening of enzyme in the compound process of preparation formula 7
[0050] Dissolve methyl 3-tert-butoxycarbonylamino-4-hydroxy-cyclohexanecarboxylate (5 g) in methyl tert-butyl ether (50 mL), add enzyme (0.1 g), vinyl acetate (2 g), 40 °C and stirred for 48 hours, the reaction results are shown in Table 2.
[0051] The resolution of the compound of formula 5 catalyzed by different hydrolases in table 2
[0052]
[0053] The results showed that porcine pancreas lipase, bovine pancreas lipase, lipase AK and lipase PS had better effects, and the de values were all greater than 96%. In terms of overall yield, when the hydrolase is selected from lipase PS, the effect is the best.
Embodiment 3
[0054] Example 3, Preparation of (1S, 3R, 4R)-3-tert-butoxycarbonylamino-4-hydroxyl-cyclohexanecarboxylic acid
[0055] 50 g of 3-tert-butoxycarbonylamino-4-hydroxyl-cyclohexanecarboxylic acid methyl ester prepared in step (4) of Example 1 was dissolved in 250 mL of ethyl acetate, and 0.83 g of bovine pancreatic lipase and 20 g of vinyl acetate were added. After stirring at 55°C for 24 hours, the reaction was terminated, the reaction solution was spin-dried, and purified by column chromatography (petroleum ether: ethyl acetate = 100:1) to obtain 19.5 g of a white solid, de = 90%.
[0056] Dissolve 10 g of white solid in 50 mL of tetrahydrofuran, add 50 mL of water and 1 g of LiOH, and react at 50° C. for 24 hours. Wash the reaction solution with ethyl acetate, continue to add an equal volume of ethyl acetate to the water phase, cool down to 0-10°C, add a certain amount of hydrochloric acid (6N), and adjust the pH to about 2. The liquid was separated, the aqueous phase was ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com