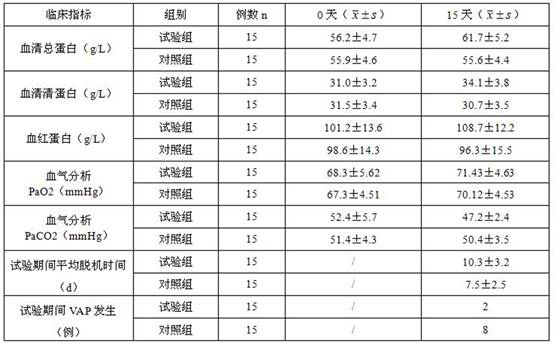
A kind of enteral nutrition composition for mechanically ventilated patients and preparation method thereof
A nutritional composition and enteral nutrition technology, applied in the direction of drug combination, active ingredients of phosphorus compounds, active ingredients of iodine compounds, etc., can solve the problems of unable to meet the characteristic nutritional needs of mechanically ventilated patients, incomplete nutrition, etc., to achieve guaranteed performance, The effect of short sterilization time and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
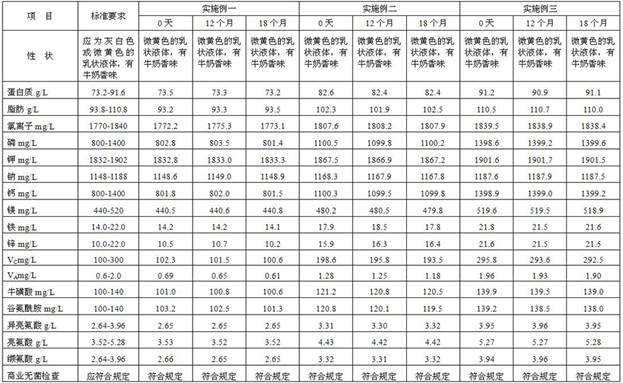
[0029] A preparation method of an enteral nutrition composition for mechanically ventilated patients of the present invention comprises the following steps: (1) oil phase preparation: under the protection of inert gas, take fat, stir, add emulsifier and vitamin Cut the fat-soluble component of the fat-soluble component for 15-20 minutes, disperse evenly, and prepare an oil phase; (2) Preparation of the water phase: Take water at 30-40 ° C, under the protection of an inert gas, add it to the vitamin while stirring After the water-soluble components, carbohydrates, proteins and minerals are completely dispersed, add taurine, L-carnitine and branched-chain amino acids, and cut for 10-15 minutes to form a water phase mixture; (3) Preparation of colostrum : Under the protection of an inert gas, while stirring, add the oil phase obtained in step (1) to the water phase mixture obtained in step (2), and cut for 20-30min to make colostrum; (4) homogeneous: The colostrum obtained in ste...
Embodiment 1
[0032] An enteral nutrition composition for mechanically ventilated patients of the present invention is prepared by the following steps.
[0033] (1) Weighing of raw materials: Weigh in order according to the prescription quantity:
[0034] ①Whey protein 36.6Kg, pea protein 22.0Kg, sea cucumber peptide 7.3Kg, fish skin collagen peptide 7.3Kg to form protein;
[0035] ② Maltodextrin 73.2Kg, seaweed polysaccharide 18.3Kg to form carbohydrates;
[0036] ③Olive oil 21.2Kg, soybean oil 21.2Kg, fish oil powder 28.2Kg, medium chain triglyceride 23.2Kg to form fat;
[0037] ④The mass concentration of the emulsifier is 0.08%, and the emulsifier is composed of 0.16Kg of monoglyceride and diglyceride, 0.24Kg of polyglycerin fatty acid ester, and 0.4Kg of lecithin;
[0038] ⑤ Disodium hydrogen phosphate 3.67Kg, sodium chloride 1.15Kg, potassium citrate monohydrate 15.2Kg, calcium carbonate 2.00Kg, magnesium sulfate heptahydrate 4.46Kg, ferrous sulfate 38.14g, zinc sulfate heptahydrate ...
Embodiment 2
[0053] An enteral nutrition composition for mechanically ventilated patients of the present invention is prepared by the following steps.
[0054] (1) Weighing of raw materials: Weigh in order according to the prescription quantity:
[0055] ①Whey protein 41.2Kg, pea protein 24.7Kg, sea cucumber peptide 16.5Kg to form protein;
[0056] ② Maltodextrin 51.2Kg, seaweed polysaccharide 12.8Kg to form carbohydrates;
[0057] ③Olive oil 23.1Kg, soybean oil 23.1Kg, fish oil powder 30.7Kg, medium chain triglyceride 25.6kKg to form fat;
[0058] ④The mass concentration of the emulsifier is 0.18%, containing 0.36Kg of monoglyceride and diglyceride, 0.54Kg of polyglycerin fatty acid ester, and 0.9Kg of lecithin to form an emulsifier;
[0059] ⑤Disodium hydrogen phosphate 5.04Kg, sodium chloride 1.17Kg, potassium citrate monohydrate 15.5Kg, calcium carbonate 2.75Kg, magnesium sulfate heptahydrate 4.87Kg, ferrous sulfate 49.03g, zinc sulfate heptahydrate 70.36g, chlorine tetrahydrate 14.42...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com