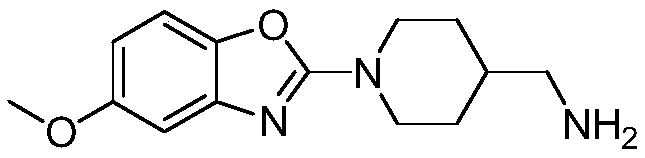
Preparation method of substituted benzoxazole derivative
A technology of oxazole and nucleophilic substitution, applied in the field of new preparation of pharmaceutical intermediates, can solve problems such as difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
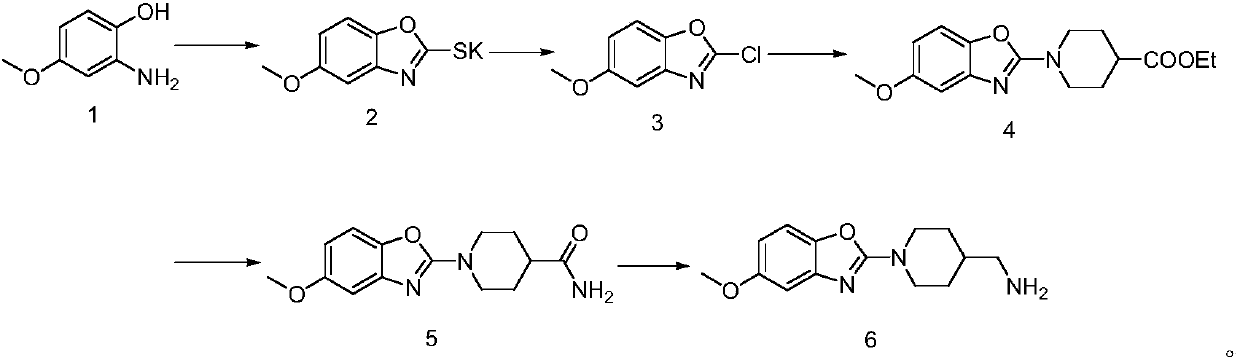
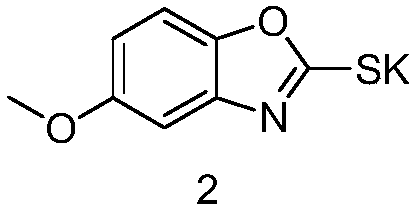
[0023] (1) Synthesis of 5-methoxybenzo[d]oxazole-2-thiolate potassium
[0024] Add 40g of 2-amino-4-methoxyphenol to 380ml of pyridine, add 63g of potassium ethylxanthate, heat under reflux and stir for 5 hours, concentrate to remove most of the pyridine, add dilute hydrochloric acid and ethyl acetate, divide The organic phase was collected, dried and concentrated, and the residue was separated on a column to obtain 51 g of potassium 5-methoxybenzo[d]oxazole-2-thiolate.
[0025] (2) Synthesis of 5-methoxy-2-chlorobenzo[d]oxazole
[0026] Add 50g of 5-methoxybenzo[d]oxazole-2-thiolate potassium to 1000ml of chloroform, cool to 0°C, pour in chlorine to saturation, keep at 0°C and stir for 2 small tests, then warm to room temperature to continue Stir for 5 hours, concentrate, add water and ethyl acetate, extract and separate, collect the organic phase, separate, dry, concentrate, and separate the residue on a silica gel column to obtain 36g of 5-methoxy-2-chlorobenzo[d ] Oxazole.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com