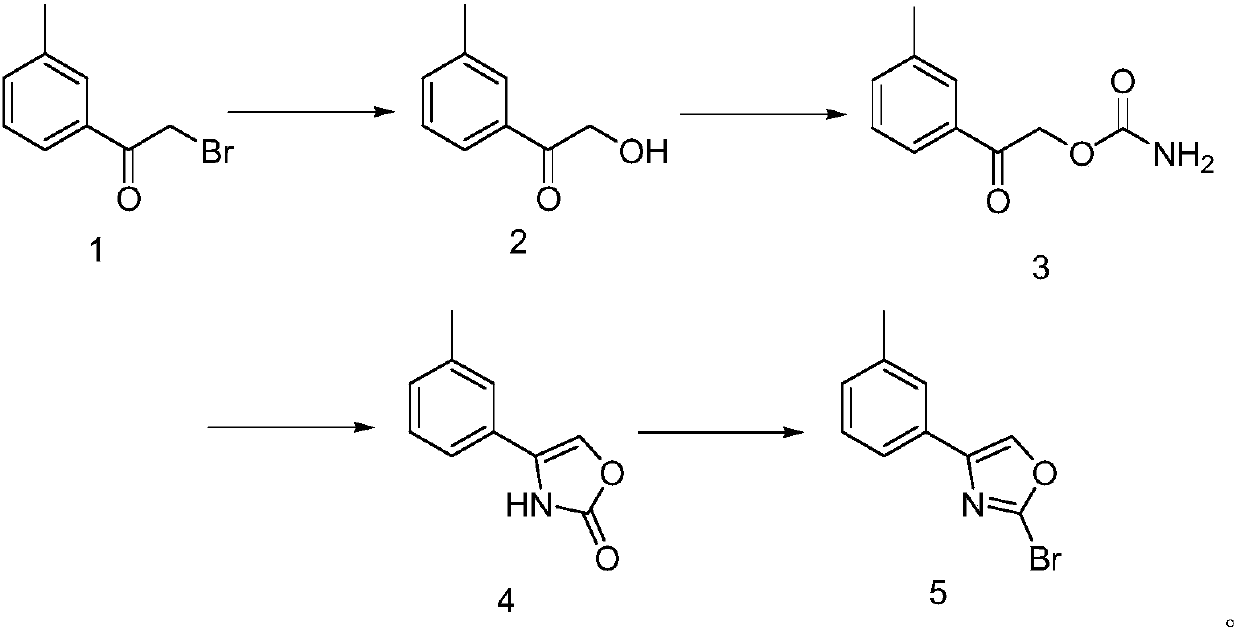
2-bromo-1-(3-methoxyphenyl)oxazole preparation method
A technology of methoxyphenyl and methylphenyl, which is applied in the field of new preparation of pharmaceutical intermediates, and can solve problems such as difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
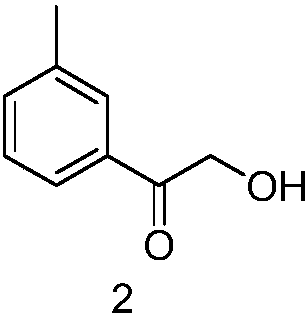
[0021] (1) Synthesis of 1-(3-methylphenyl)-2-hydroxyethanone
[0022] Add 50g of 2-bromo-1-(3-methylphenyl)ethanone to 600ml of ethanol, add 100ml of water and 80g of sodium hydroxide, heat to reflux for 20 hours, cool, concentrate and then add ethyl acetate, separate liquid, After drying and concentration, the residue was subjected to column separation to obtain 29 g of 1-(3-methylphenyl)-2-hydroxyethanone.
[0023] (2) Synthesis of carbamic acid-(1-(3-methylphenyl)-2-hydroxyethanone) ester
[0024] Add 51g of N,N-dimethylaniline to 220ml of toluene, add 110ml of 1.9M phosgene toluene solution, cool to 0°C, add 28g of 1-(3-methylphenyl)-2-hydroxyethanone, and stir After 30 min, 600 ml of ammonia water was added, stirred for 1 h, filtered, and the filter cake was collected and dried to obtain 32 g of carbamic acid-(1-(3-methylphenyl)-2-hydroxyethanone) ester.
[0025] (3) Synthesis of 4-(3-methylphenyl)oxazol-2-(3H)-one
[0026] Add 30g of carbamic acid-(1-(3-methylphenyl)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com