Antibody production method
A production method and antibody technology, applied in chemical instruments and methods, peptides, specific peptides, etc., can solve the problems of limited adjustment range of charge variants, poor versatility, and no significant increase in antibody yield, to increase antibody yield and reduce acidity. Peak content, the effect of improving charge heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The antibody production process of this embodiment is as follows:
[0045] (a) Add basal culture medium to the cell culture flask, inoculate the GS-CHO antibody-expressing cell line, and carry out routine culture at 37°C after inoculation;
[0046] (b) After culturing for a certain period of time (the 4th day), the culture temperature was lowered to 34°C; at the same time, on the 4th day, the following strategy was used to add the culture solution containing the protein hydrolyzate until the end of the culture:
[0047] Once every two days, the volume of the fed-batch culture solution added each time is 5%, 2%, 5%, 2%, 5% of the culture volume;
[0048] c) Stop the cell culture when the cell viability drops to 50% or the cell density is lower than 1 / 3 of the highest cell density, and harvest the supernatant.
[0049] in,
[0050] The basic culture medium is Dynamis;
[0051] Fed-batch medium is Feed C;
[0052]The inoculated antibody expression cell lines were GS-CH...
Embodiment 2
[0062] The present embodiment adopts the method identical with embodiment 1 to cultivate, wherein,
[0063] The present embodiment selects 6 kinds of basic culture fluids as Dynamis, T54, Cellvento TM CHO-210, Cellvento TM CHO-200, ApmliCHO CD, OPM-CD11V;
[0064] Fed-batch medium is Feed C;
[0065] The protein hydrolyzate is soybean protein hydrolyzate, and the added concentration is 100g / L;
[0066] In this embodiment, the fed-batch culture solution without protein hydrolyzate was also used as a control group;
[0067] The inoculated cell line is GS-CHO cell antibody expression strain 2, and the cell inoculation density of the antibody expression cell line is (0.5*10 6 cells / ml);
[0068] The culture product was tested and the following results were obtained:
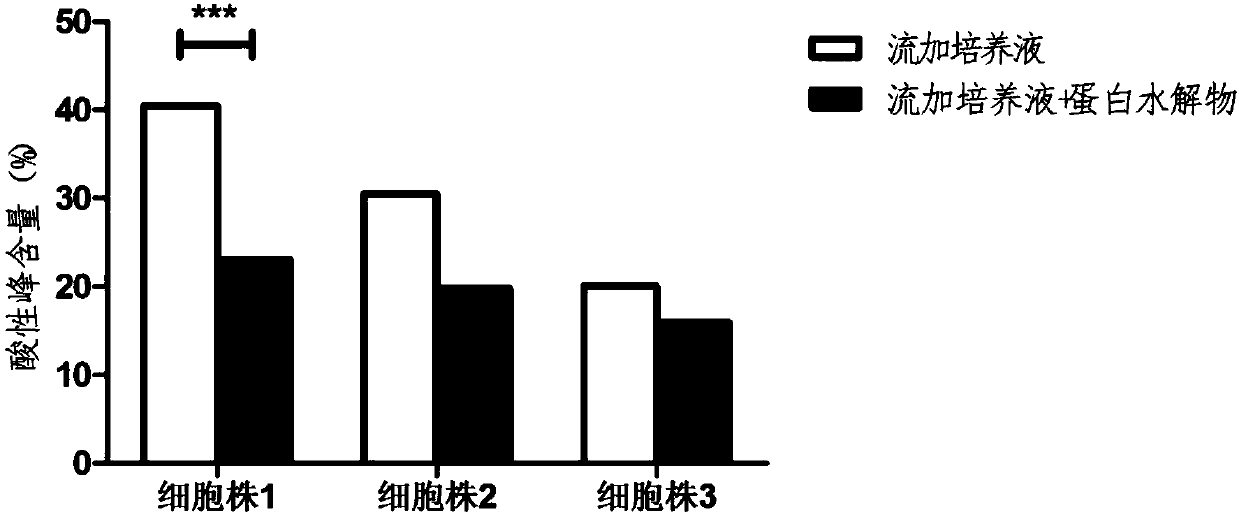
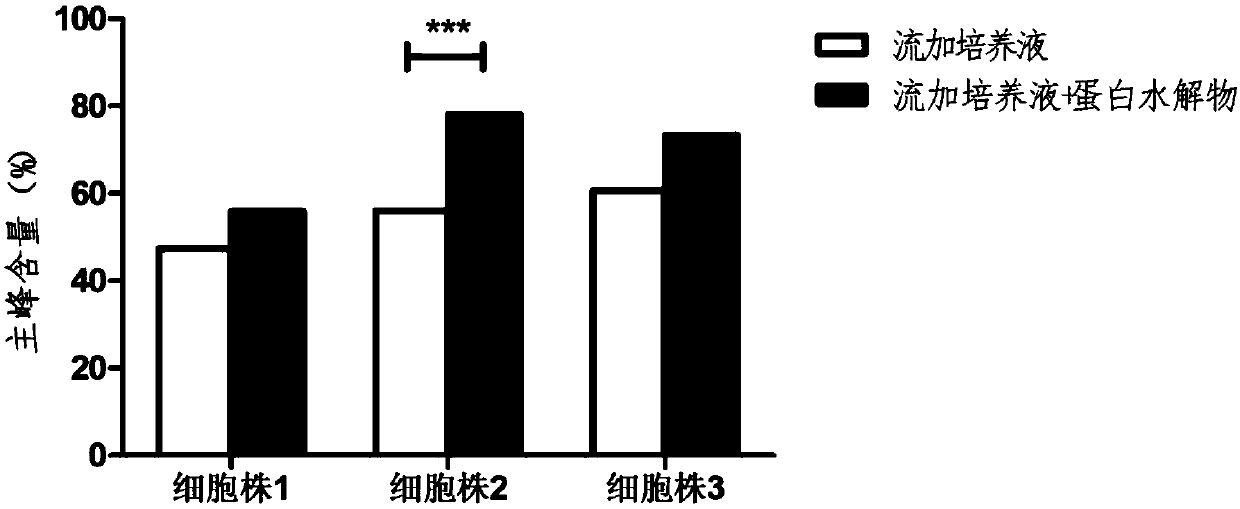
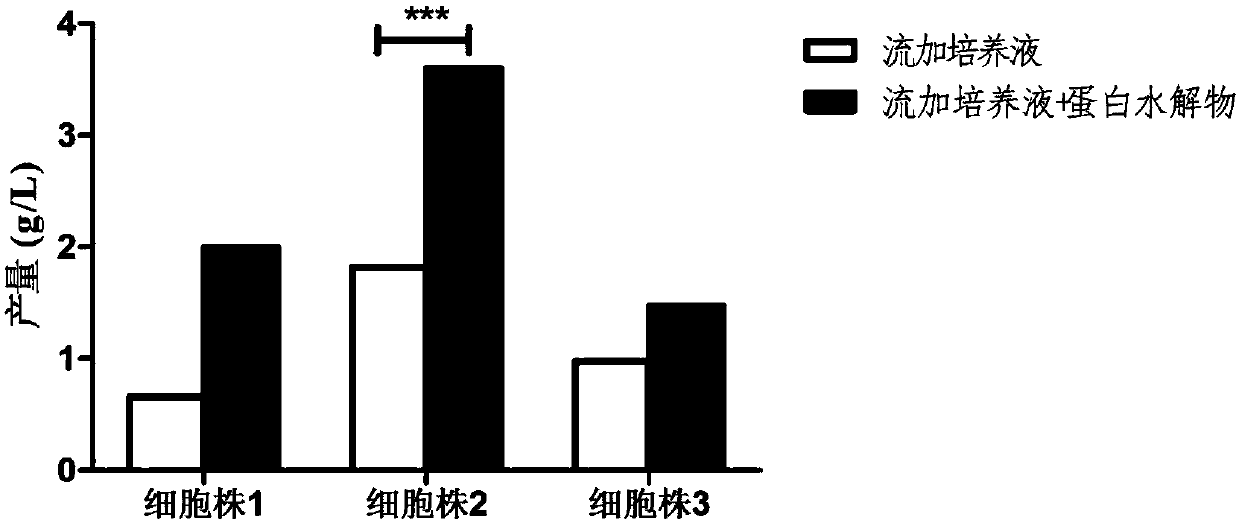
[0069] See Figure 4-Figure 6 , where, compared to the control group,
[0070] Using the culture medium Dynamis, the acidic peak content of the antibody decreased from 23.4% to 19.6%, the main peak content i...
Embodiment 3
[0078] The present embodiment adopts the method identical with embodiment 1 to cultivate, wherein,
[0079] The basal culture medium selected in this embodiment is Dynamis;
[0080] Fed-batch medium is Feed C;
[0081] The protein hydrolyzate is corn protein hydrolyzate, and the added concentration is 20g / L, 40g / L, 60g / L, 100g / L;
[0082] In this embodiment, the fed-batch culture solution without protein hydrolyzate was also used as a control group;
[0083] The inoculated cell line is GS-CHO antibody-expressing cell line 2, and the cell inoculation density of the antibody-expressing cell line is (0.5*10 6 cells / ml).
[0084] The culture product was tested and the following results were obtained:
[0085] See Figure 7-Figure 9 , where, compared to the control group,
[0086] When the concentration of protein hydrolyzate was 20g / L, the acidic peak content of the antibody was 19.8%, the main peak content was 72.1%, and the yield was 1.53g / L;
[0087] When the concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com