A process for the preparation of bis(chloromethyl)dichlorosilane and bis(chloromethyl)(aryl)chlorosilane
一种二氯硅烷、氯甲基的技术,应用在制备双二氯硅烷和双氯硅烷领域,能够解决低产率、高毒性副产物、危险化学品等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0060] The following examples illustrate the invention, but are not intended to limit the scope of the invention. The examples of the present invention demonstrate that the methods for preparing BCMCS and / or BCMCS according to the present invention can safely produce BCMCS and / or BCMCS at low cost while producing high yields.
example 1
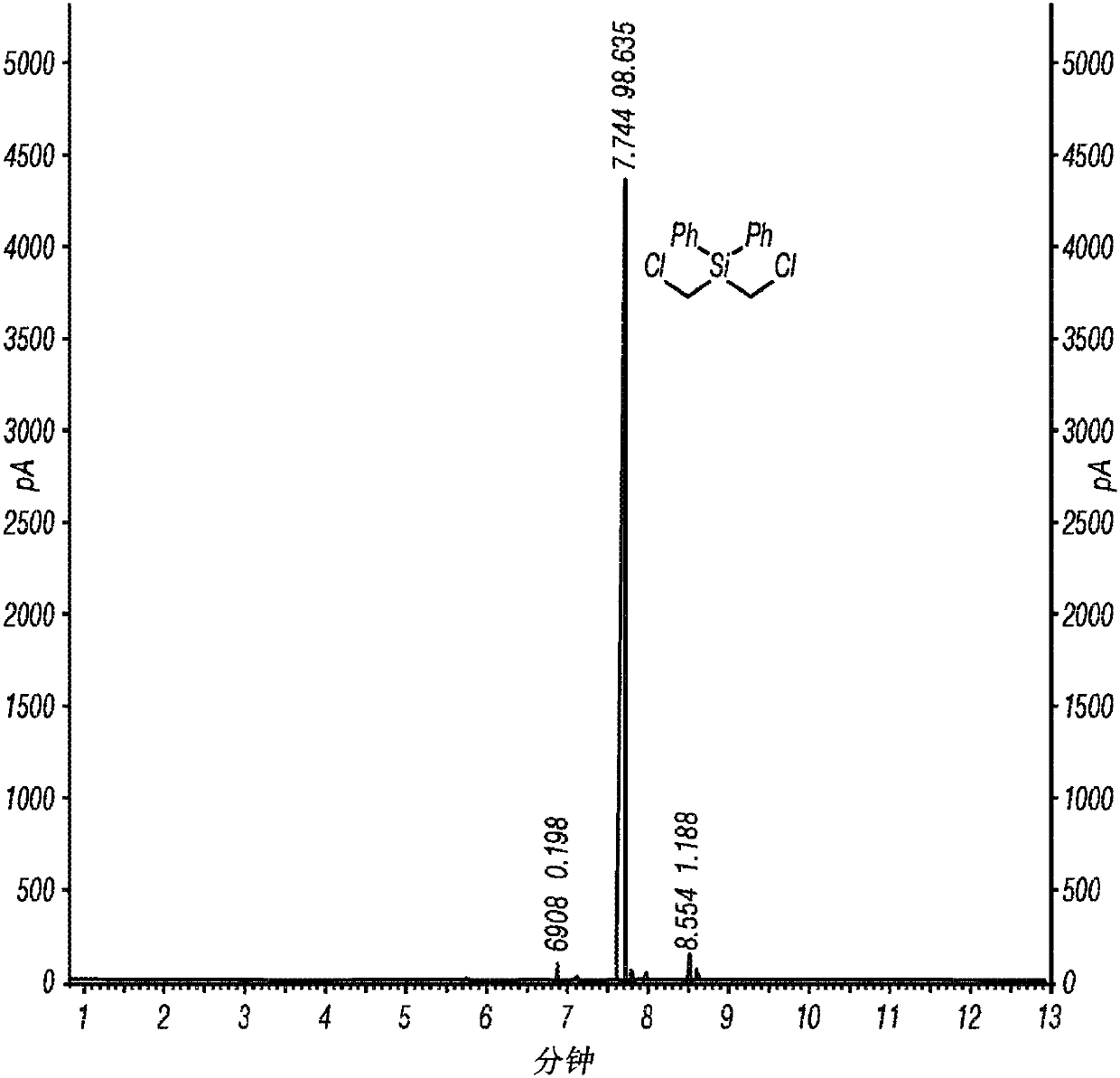
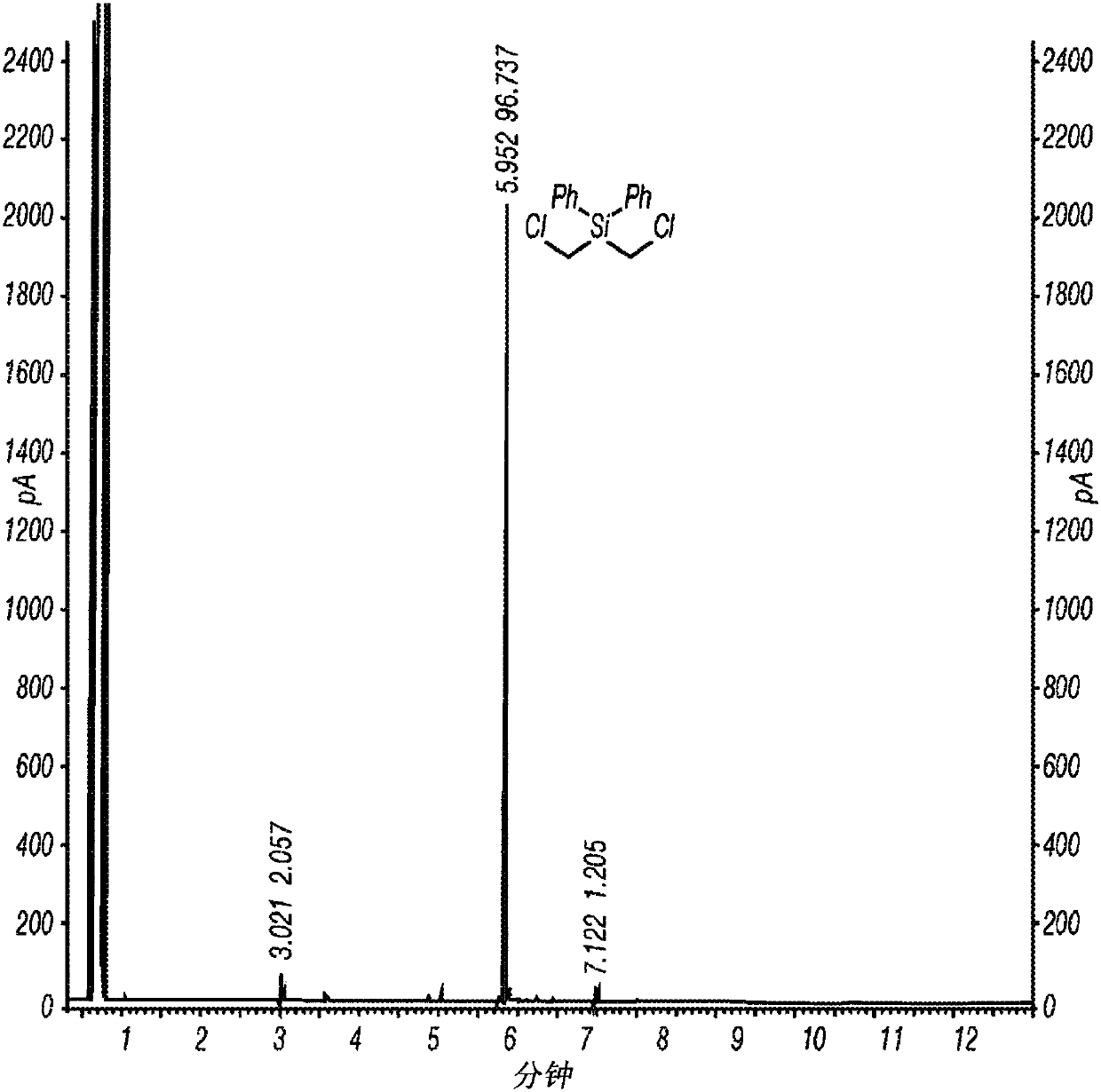
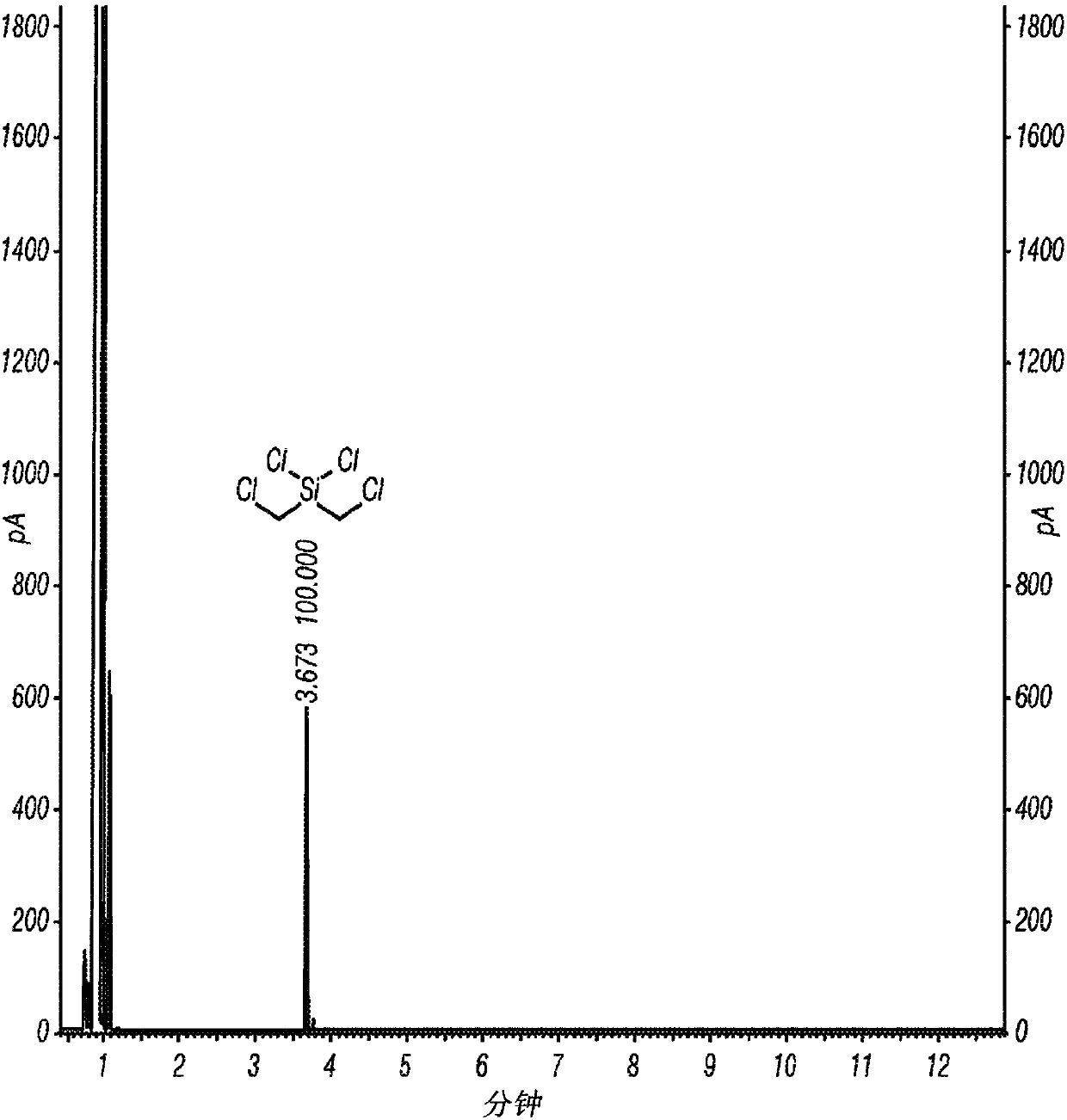
[0062] Inventive Example 1 was prepared according to the following procedure. A 1 L three neck round bottom flask equipped with overhead stirrer, thermometer and nitrogen pad was charged with AlCl 3 (33.5 g, 250 mmol) and dry hexane (450 mL). The reaction mixture was cooled to near or at or near 0°C in an ice bath and bis(chloromethyl)diphenylsilane (28.1 g, 100 mmol) was added at 0°C followed by acetyl chloride (18 mL) in an ice bath , 250 mmol) in hexane (50 mL). The reaction mixture was stirred in an ice bath for 4 hours. sampling 1 H NMR analysis, which indicated the formation of the intermediate (ClCH) as the only product 2 ) 2 Si(Ph)Cl. The reaction mixture was warmed to ambient temperature and stirred until all (ClCH) 2 ) Si(Ph)Cl is converted to the desired product (ClCH 2 ) 2 SiCl 2 (overnight). The resulting reaction mixture was filtered under nitrogen atmosphere. The wet cake was washed with dry hexane (100 mL). The filtrate was concentrated under reduc...
example 2
[0064] Inventive Example 2 was prepared according to the following procedure. Charge AlCl to a 2L three-neck round bottom flask equipped with overhead stirrer, thermometer and nitrogen pad 3(105.5 g, 791.2 mmol) and anhydrous hexane (900 mL). The reaction flask was cooled in a cold water bath, and bis(chloromethyl)diphenylsilane (89.02 g, 316.5 mmol) was added at 20°C. After the addition of bis(chloromethyl)diphenylsilane was complete (20 minutes), acetyl chloride (56.2 mL, 791.2 mmol) was added via a syringe pump at 20 °C over 3 hours. The reaction mixture was stirred overnight at 20°C to 25°C and then warmed to 30°C to 35°C until the 1 H NMR monitored the reaction to be complete (3 hours). The reaction mixture was cooled to ambient temperature and filtered under nitrogen atmosphere. The solid was washed with hexanes (3 x 100 mL). Concentration of the filtrate with a rotary evaporator under reduced pressure afforded the desired product, (ClCH 2 ) 2 SiCl 2 , 56.46 g (9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com