A mutant of β-galactosidase and its preparation method and application
A galactosidase and mutant technology, applied in the field of genetic engineering and enzyme engineering, can solve the problems of limited application, yield of only 19%, poor probiotic effect, etc., and achieve the effect of strong transglycoside activity and high industrial production value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of single mutant and wild enzyme
[0043]The β-galactosidase gene was connected with pMD19-T simple to obtain the AorE / pMD19-T simple plasmid, which was used as a template and Sac1m-F and Sac1m-R were used as primers for mutation. The above PCR product was digested with Dpn1, and the resulting plasmid was transformed into Escherichia coli JM109 competent cells to obtain the AorE-M / pMD19-T simple plasmid.
[0044] Sac1m-F: TCCACAAGATC AGG GCTCTTGGTTTCAAC (the mutation site is underlined)
[0045] Sac1m-R: GTTGAAACCAAGAGC CCT GATCTTGTGGA (the mutation site is underlined)
[0046] 1. Preparation of wild enzyme: AorE-M / pMD19-T simple plasmid and pPIC9k vector were digested with Snab1 and Not1, the digested product was ligated with T4 ligase, the ligated product was transferred into Escherichia coli JM109 competent cells, and the bacteria were picked The plasmid was extracted, and the plasmid was named AorE-M / pPIC9k.
[0047] Preparation ...
Embodiment 2
[0060] Example 2: Construction of double mutants.
[0061] Using the plasmid of the mutant N140C constructed in Example 1 as a template for double mutation, and according to the primers for site-directed mutation designed in Example 1, using rapid PCR technology, the plasmid carrying the gene encoding the mutant N140C was subjected to site-directed mutation to construct Double mutants N140C / F264W and N140C / W806F. Sequence separately to confirm whether the coding gene of the β-galactosidase double mutant is correct, and carry out Snab1 and Not1 double digestion of the plasmid with the correct sequencing result and the pPIC9k vector, and connect the digested product with T4 ligase, and transfer the ligated product into the large intestine Bacillus JM109 competent cells were picked to extract the correct plasmid and transformed into yeast KM71 cells to obtain recombinant bacteria AorE-M / pPIC9k-N140C / F264W and AorE-M / pPIC9k-N140C / W806F capable of expressing double mutant genes.
Embodiment 3
[0062] Example 3: Preparation of Wild Bacteria and Mutant β-galactosidase Enzyme Solution
[0063] The recombinant bacteria AorE-M / pPIC9k / KM71, AorE-M / pPIC9k-N140C, AorE-M / pPIC9k-F264W, AorE-M / pPIC9k-F304Q, AorE-M / pPIC9k-W806F, AorE-M / pPIC9k-N140C / F264W and AorE-M / pPIC9k-N140C / W806F were respectively transferred to BMGY liquid medium, and cultured at 30°C for 24 hours to obtain the inoculum solution. The above bacterial liquid was centrifuged, all the bacterial cells were transferred into 50 mL BMMY liquid medium, cultured at 30° C. for 5 days, and the final concentration of 0.75% methanol was added every 24 hours to induce enzyme production. The fermented liquid is centrifuged, and the supernatant is crude enzyme liquid.
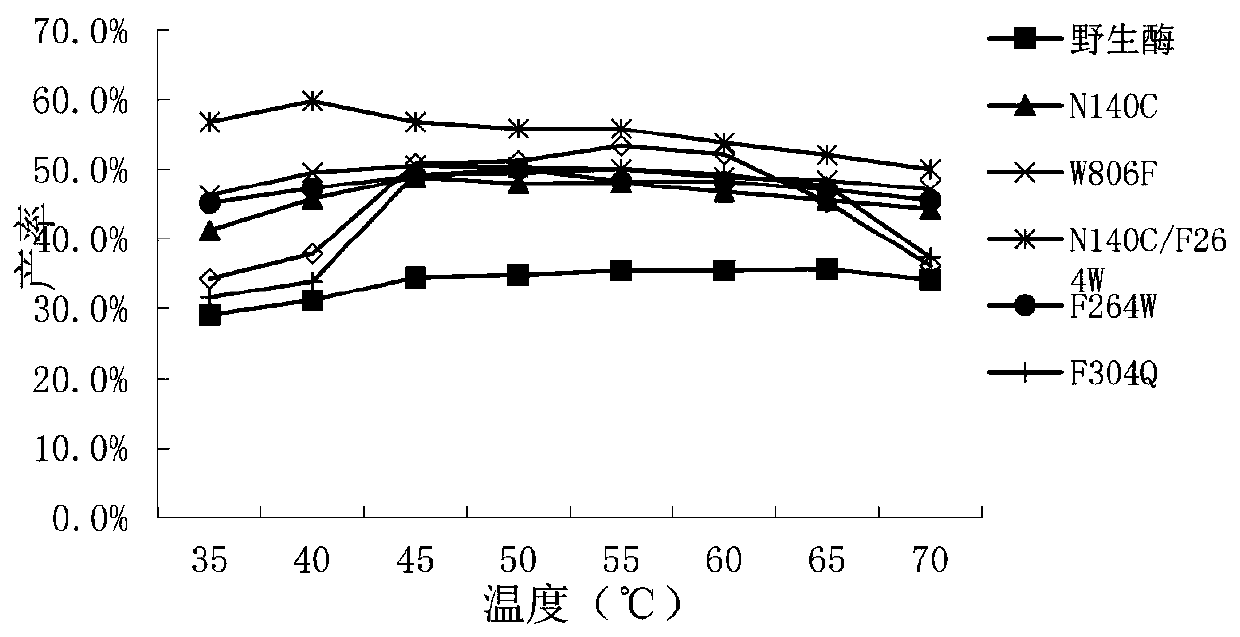
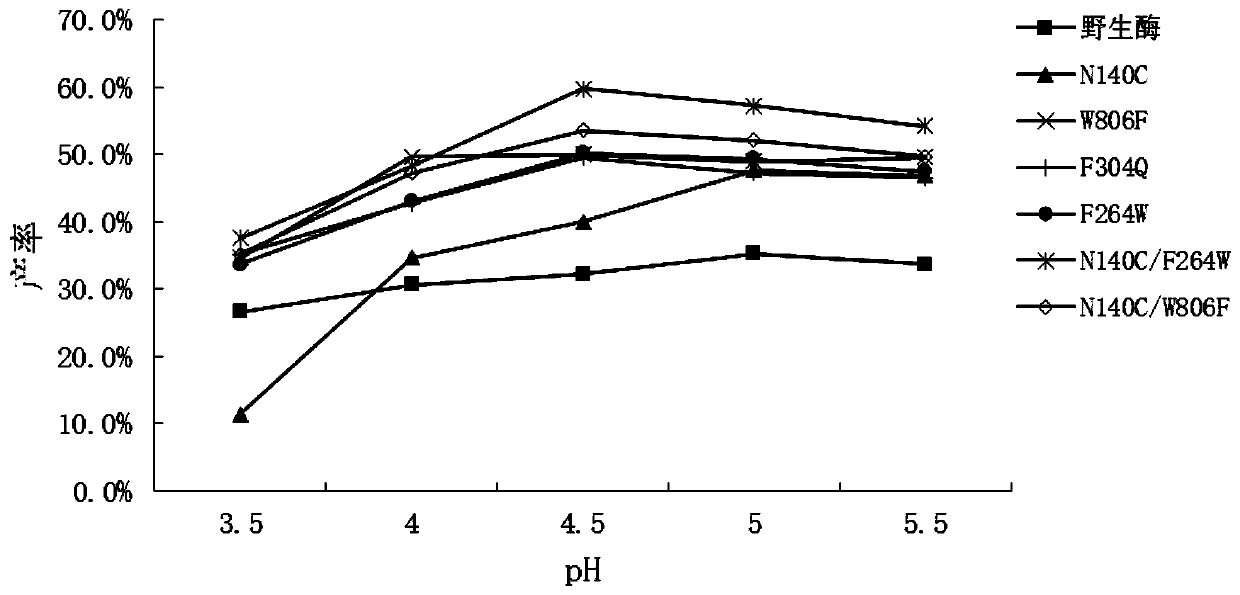
[0064] The enzymatic activity of the crude enzyme solution was determined, and the ONPG hydrolase activity of wild-type β-galactosidase (WT) and mutants is listed in Table 1.
[0065] Table 1 Enzyme activity of wild-type β-galactosidase and mutant enzyme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com