A kind of myceliophthora thermophila exoglucosidase mutant and its application
A technology of glucosidase and recombinant bacteria, applied in the field of genetic engineering, can solve problems such as difficult to meet industrial production, cellulase activity or low yield, and achieve improved catalytic efficiency, reduced inhibition, and good thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Construction of Exoglucosidase CBH1 of Family VII of Myceliophthora thermophila and Deletion Mutants
[0038] (1) Cloning of cbh1 gene of family 7 exoglucanase of Myceliophthora thermophila
[0039] Inoculate Myceliophthora thermophila (preservation number: CGMCC NO.3.17368) into carboxymethylcellulose induction medium, culture at 50°C for 3 days, scrape mycelia to extract total RNA, and perform reverse transcription to obtain cDNA. Primers were designed according to the nucleotide sequence of exoglucanase CBH1 of the seventh family of Myceliophthora thermophila (GenBank: XM_003660741.1), and EcoR I and Not I restriction sites (underlined) were inserted into the upstream and downstream primers respectively Mark), add 6 histidine (His) tags (dotted line mark) downstream, and design primers as follows:
[0040]cbh1-F:GCTTACGTA GAATTC CAGAACGCCTGCACTCTGAC; (SEQ ID NO. 3)
[0041] cbh1-R:TTAATTC GCGGCCGCATGATGATGATGATGATG CAGGCACTGCG; (SEQ ID NO.4)
[0042...
experiment example 2
[0053] Experimental Example 2: Transformation and Screening of Recombinant Expression Vectors
[0054] The recombinant expression vector and mutant constructed in Example 1 were digested with Pme I, and the digested product was transferred into Pichia pastoris GS115 by electric shock. The electric shock method was an existing conventional method, and the transformed product was placed on MD medium. Cultivate at 28°C, and screen with different concentrations (1-4mg / mL) of G418 after a single spot grows, select a single spot with better growth, and verify it by sequencing with universal primers 3'AOX1 and 5'AOX1.
experiment example 3
[0055] Experimental Example 3: Induced Expression of Recombinant Pichia pastoris Engineering Bacteria and Separation and Purification of Enzyme Protein
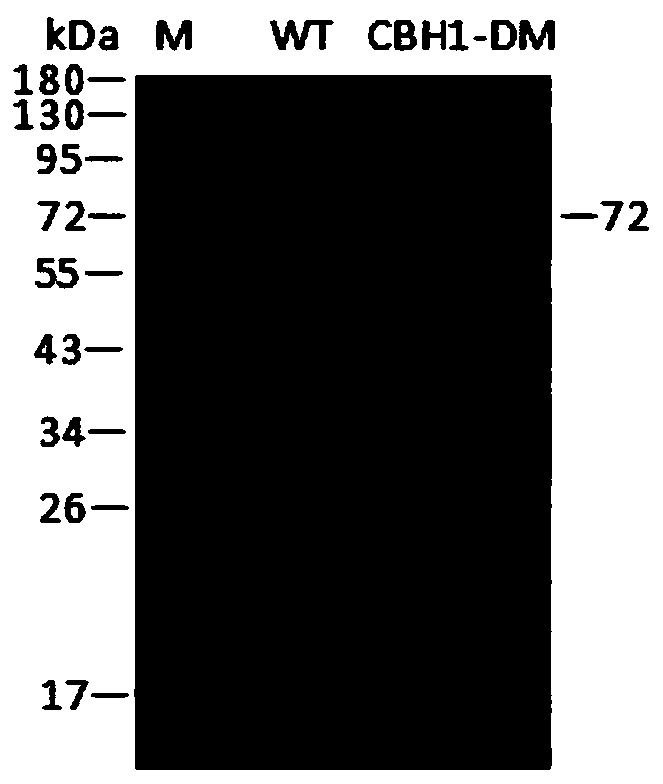
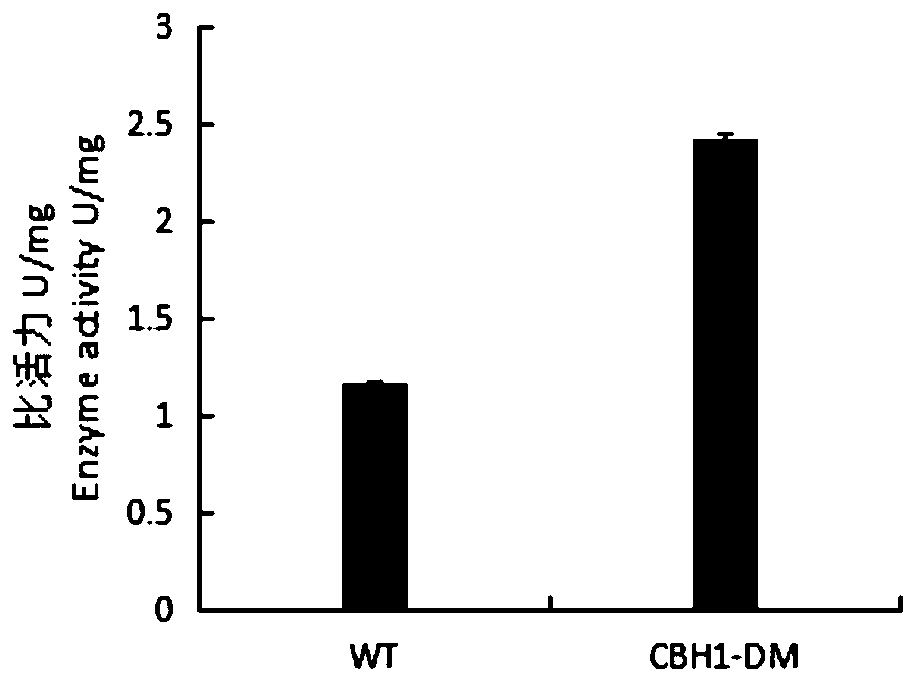
[0056] Inoculate Pichia pastoris engineering strains with correctly sequenced wild enzymes and mutated enzymes in BMGY medium, culture at 28°C, 200rpm for 24h, centrifuge at 4000rpm for 5min, collect the bacteria and transfer them to BMMY medium, at 28°C, 200rpm Under methanol induction culture. After induction of expression for 120 hours, centrifuge at 4°C and 8000rpm for 15min to obtain the supernatant, add ammonium sulfate to the supernatant to a saturated concentration, stay at 4°C overnight, centrifuge at 4°C and 8000rpm for 15min to obtain the cells, and use an appropriate amount of PBSA (pH=7.4 ) buffer and purified by HisTrap HP to obtain purified wild enzyme (WT) and mutant enzyme (CBH1-DM). Run SDS-PAGE electrophoresis for the purified wild enzyme and mutant enzyme.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com