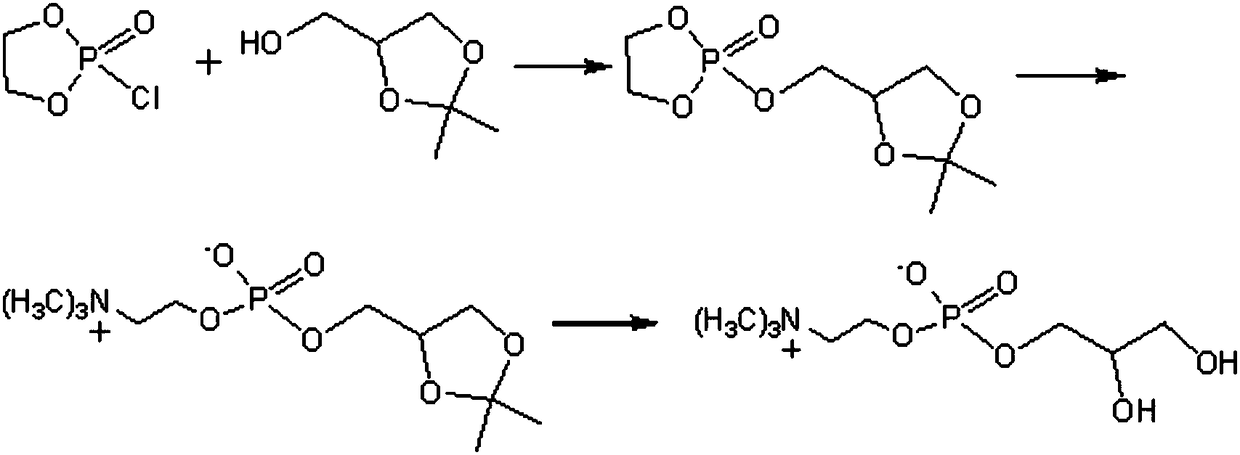
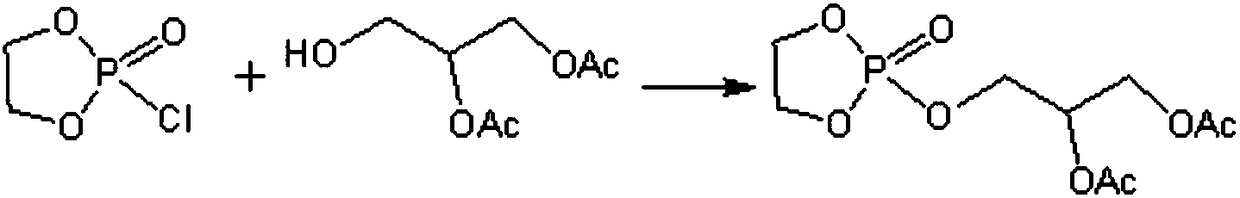
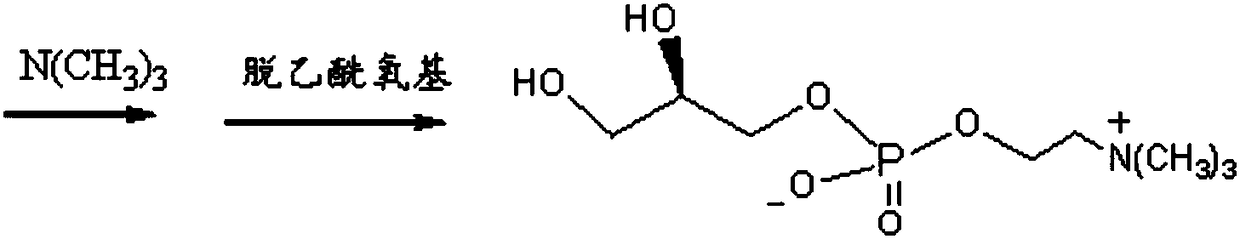
Method of preparing L-alpha-glycerol phosphatidylcholine
A technology of glycerol phosphatidylcholine and phosphorylcholine, which is applied in the field of medicine and chemical industry, can solve the problems of low yield and long reaction time of L-α-alphaphosphocholine, and achieve easy industrial production, high purity, The effect of high total reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of (R)-2-methoxy-4-chloromethyl-1,3-dioxocyclopentane.
[0076] In a 500mL glass bottle, weigh 115.5g (1.0 mole) of (R)-3-chloroglycerol, 138.0g (1.3 mole) of trimethyl orthoformate, and 19.0g p-toluenesulfonic acid. After mixing, stir and heat to React at 70-90°C. During the reaction, low-boiling materials are distilled from the distillation head, mainly methanol and a small amount of methyl formate. The progress of the reaction was detected by GC, and the reaction was complete in about 6 hours. After the reaction is complete, cool to room temperature, add sodium methoxide to neutralize to pH=6-7. First heat and depressurize to recover the low boiling point solvent, and then collect the 102-105°C fraction at -0.096Mpa to obtain 143.4g of the product with a yield of 91%.
Embodiment 2
[0078] Preparation of (R)-2-ethoxy-4-chloromethyl-1,3-dioxocyclopentane.
[0079] In a 500mL glass bottle, weigh 115.5g (1.0 mole) of (R)-3-chloroglycerol, 192.5g (1.3 mole) of triethyl orthoformate, and 19.0g p-toluenesulfonic acid. After mixing, stir and heat to React at 70-90°C. During the reaction, low-boiling materials are distilled from the distillation head, mainly ethanol and a small amount of ethyl formate. The progress of the reaction was detected by GC, and the reaction was complete in about 8 hours. After the reaction is complete, cool to room temperature, add sodium ethoxide to neutralize to pH=6-7. First heat and depressurize to recover the low boiling point solvent, and then collect the 114-116°C fraction at -0.096Mpa to obtain 154.5g of the product with a yield of 90%.
Embodiment 3
[0081] Preparation of (R)-2-methoxy-4-bromomethyl-1,3-dioxocyclopentane.
[0082] In a 500mL glass bottle, weigh 199.0g (1.0 mole) of (R)-3-bromoglycerol, 138.0g (1.3 mole) of trimethyl orthoformate, and 19.0g p-toluenesulfonic acid. After mixing, stir and heat to React at 70-90°C. During the reaction, low-boiling materials are distilled from the distillation head, mainly methanol and a small amount of methyl formate. The progress of the reaction was detected by GC, and the reaction was complete in about 6 hours. After the reaction is complete, cool to room temperature, add sodium methoxide to neutralize to pH=6-7. First heat and depressurize to recover the low boiling point solvent, and then collect the 68-73°C fraction at about 100pa to obtain 177.8g of product with a yield of 88%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap